Table 1.
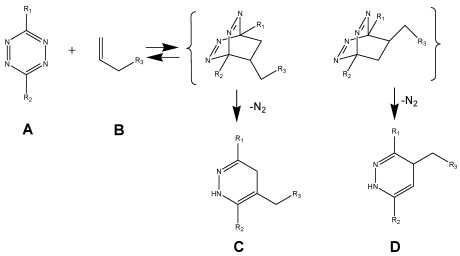
(Scheme 1) The Diels-Alder-Reaction with inverse electron demand (DARinv) is shown. R1 and R2 represent different functional moieties harbouring -I and/or -M effects on the diene A., which induce a decrease of the electron density of the tetrazine ring. Whereas in contrast the R3 features a +I effect resulting in a relative high electron densitiy in the dienophile compound. The stepwise reaction from A and B results in the stereoisomers C and D, which can be attributed to the two different variants of intermediates (bracketed) after elimination of molecular nitrogen. As shown here the reverse reaction is impossible.