Figure 2.
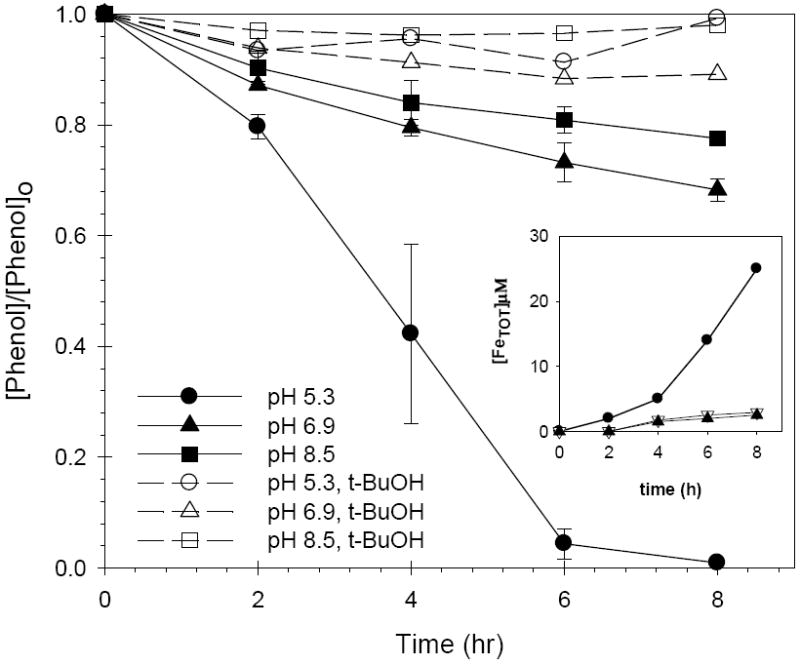
Effect of pH on phenol loss in the FeAlSi-ox/H2O2 system in the absence (solid lines) and presence (dashed lines) of t-BuOH; [phenol]o = 0.5 mM; [H2O2] = 50 mM; [FeAlSi-ox ] = 3 g/L; [t-BuOH] = 200 mM. [FeTOT] as a function of time (inset): (●) pH 5.3; (∇) pH 6.9; (▲) pH 8.5. For the purpose of clarity, error bars were eliminated from the data in the inset. In all cases, the pH decreased by less than 1 unit during the reaction.
