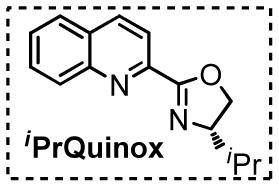
Table 1.
Optimization of reaction conditions.
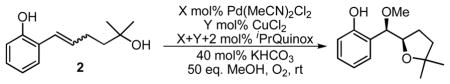 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | X | Y | solvent | time | %conva | %yielda | erb | drb |
| 1c | 10 | -- | MeOH | 15 h | 100 | 2 | 88:12 | 2.4:1 |
| 2 | 10 | -- | MeOH | 15 h | 74 | 8 | 92:8 | 2.7:1 |
| 3 | 10 | 20 | MeOH | 10 min | 100 | 95 | 92:8 | 9.4:1 |
| 4 | 4 | 8 | MeOH | 30 min | 100 | 87 | 92:8 | 9.6:1 |
| 5 | 4 | 8 | THF | 2 h | 79 | 54 | 98:2 | 7.8:1 |
| 6 | 4 | 8 | toluene | 2 h | 96 | 68 | 97:3 | 5.1:1 |
| 7 | 4 | 8 | 1:1 THF:toluene | 5 h | 100 | 67 | 97:3 | 6.7:1 |
| 8d | 4 | 8 | 1:1 THF:toluene | 2 h | 99 | 80 | 98:2 | 8.9:1 |
Reactions run on 0.1 mmol scale with [2]=0.1 M.
Determined by GC analysis using an internal standard.
Determined by GC with a column equipped with a chiral stationary phase.
With 50 mg 3 Å MS and without KHCO3.
CuCl was used in place of CuCl2