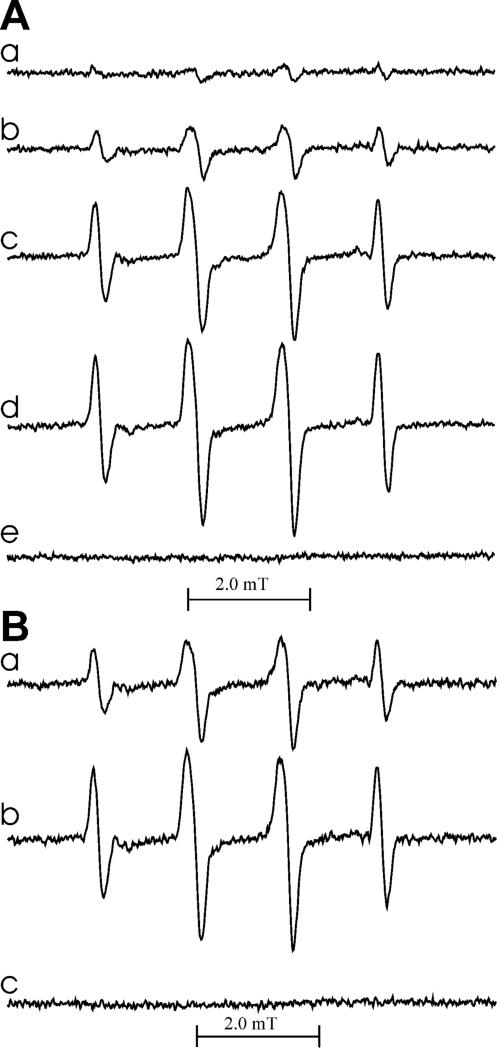
Figure 7.
EPR spectra of DMPO/•SG adducts generated by oxidation of salbutamol (A) and fenoterol (B) by MPO/H2O2 in the presence of glutathione. The reaction was carried out in 50 mM phosphate buffer (pH 7.0) containing DTPA (100 μM), DMPO (18 mM), GSH (4 mM), and MPO (0.01 U/250 mL). Panel A: spectra observed in the presence of salbutamol (0, 80, 400 and 800 μM for spectra a-d, respectively). Spectrum e was obtained in the absence of GSH but with 800 μM salbutamol. Panel B: spectra observed in the presence of fenoterol (2 and 20 μM for spectra a and b, respectively). Spectrum c was obtained in the absence of GSH but with 400 μM fenoterol present. The reaction was initiated by the addition of H2O2 to a final concentration of 84 μM. The recording was started 1 min after H2O2 addition. The spectra shown are average of 5 scans and represent a typical result.