Abstract
Objectives:
Children with adenotonsillar hypertrophy and those with an abnormal craniofacial morphology are predisposed to having sleep disordered breathing; many of these children are mouth breathers. The aim of this study was to determine whether an association exists between polysomnographic findings and cephalometric measures in mouth-breathing children.
Methods:
Twenty-seven children (15 mouth-breathing children and 12 nose-breathing children [control subjects]), aged 7 to 14 years, took part in the study. Polysomnographic variables included sleep efficiency, sleep latency, apnea-hypopnea index, oxygen saturation, arousal index, number of periodic limb movements in sleep, and snoring. Cephalometric measures included maxilla and mandible position, occlusal and mandibular plane inclination, incisor position, pharyngeal airway space width, and hyoid bone position.
Results:
As compared with nose-breathing children, mouth breathers were more likely to snore (p < 0.001) and to have an apnea-hypopnea index greater than 1 (p = 0.02). Mouth-breathing children were also more likely to have a retruded mandible, more inclined occlusal and mandibular planes, a smaller airway space, and a smaller superior pharyngeal airway space (p < 0.01). The apnea-hypopnea index increased as the posterior airway space decreased (p = 0.05).
Conclusions:
Our study showed an association between polysomnographic data and cephalometric measures in mouth-breathing children. Snoring was the most important variable associated with abnormal craniofacial morphology. Orthodontists should send any mouth-breathing child for an evaluation of sleep if they find that the child has a small superior pharyngeal airway space or an increased ANB (the relationship between the maxilla and mandible), NS.PlO (occlusal plane inclination in relationship to the skull base), or NS.GoGn (the mandibular plane inclination in relation to the skull base), indicating that the child has a steeper mandibular plane.
Citation:
Juliano ML; Machado MAC; de Carvalho LBC; Zancanella E; Santos GMS; do Prado LBF; do Prado GF. Polysomnographic findings are associated with cephalometric measurements in mouth-breathing children. J Clin Sleep Med 2009;5(6):554-561.
Keywords: Sleep disordered breathing, polysomnography, lateral radiography, mouth-breathing children
Polysomnography, the gold-standard test for making the diagnosis of sleep disordered breathing (SDB), has been used for more than 30 years and is accepted as the most comprehensive and accurate method of determining the presence and severity of obstructive sleep apnea syndrome (OSAS).1 OSAS is characterized by repetitive episodes of partial or complete airway obstruction during sleep, which may or may not be associated with hypoxemia and sleep fragmentation.1,2 In children, adenoid and tonsillar hypertrophy may cause nasal and pharyngeal obstruction,3 preventing the child from adequately breathing through the nose and forcing the child to breathe through the mouth during both sleep and wakefulness.4,5 This obstruction is the main etiologic factor in OSAS in children.
In children without respiratory disorders, nasal breathing leads to correct craniofacial growth and adequate development of interaction with other functions, such as chewing and swallowing.5 Mouth breathing, on the other hand, is an important cause of abnormal craniofacial development,5 including dental malocclusion, an increase in the anterior and inferior facial height, a narrowing and deepening of the palate, a tendency to develop an open bite or crossbite, protrusion of the upper incisors, and changes in the head position relative to the neck.6–10
The lateral cephalogram, a standardized skull radiograph taken with the patient's head in the natural position, is used throughout the world to analyze both bony and soft tissue craniofacial relationships and has been used to determine craniofacial morphology in adults11,12 and children13–15 with OSAS. Cephalometry is also a useful tool to evaluate anatomic abnormalities, follow craniofacial growth, and develop orthodontic and facial orthopedic treatment plans. Most orthodontists use some type of cephalometric analysis before developing any orthodontic treatment plan and, therefore, have lateral teleradiography readily available. In addition to supporting the diagnosis of dental occlusion or in identifying changes in dental occlusion or the skull, cephalometry is also an important resource to evaluate the nasopharyngeal airway space and, thus, to assess patients with OSAS.13,14,16 Currently, however, the use of cephalometry to evaluate patients for orthodontic treatment does not include an assessment of the airway space.
Although the medical literature supports the use of polysomnography and teleradiography to assess patients with SDB, clearly defined variables that should be used in this assessment have not been elucidated. It is also not clear whether specific teleradiographic parameters are predictive of polysomnographic findings, especially in children with OSAS.
Our hypothesis is that angle and linear measurements might be predictive of abnormal polysomnographic variables in children with SDB, i.e., cephalometry might help the orthodontist to identify findings that indicate that a child has SDB. The objective of this study is, therefore, to compare polysomnographic and cephalometric data of nose- and mouth-breathing children to investigate possible associations.
MATERIAL AND METHODS
Population and Setting
We evaluated 27 children (15 mouth breathers and 12 nose breathers) who were 7 to 14 years of age (mean age 10.3 years). To avoid selection bias that may have occurred had we recruited our sample from only a specialized health-care center (i.e., the neurology department based at the Federal University of São Paulo), 1 author (MLJ) assessed 25 children from Jardim Colonial, a community center in São Paulo, Brazil, where only basic health care is provided (primary-care setting). This community center provides recreation activities to keep children off the streets, and the authors used this opportunity to recruit children from the general population. Parents were informed that taking part in this study was an opportunity for their children to receive health care from a specialized center. In the nose-breathing group (12 participants), we included 10 children from Jardim Colonial and 2 children who had been referred to our sleep clinic for assessment of a parasomnia. Parents were informed of the study's objectives and signed the informed consent form to carry out teleradiography and polysomnography before any intervention took place. For those recruited from Jardim Colonial, this consent process took place while the parents and children were at the community center. This study was approved by the Ethics Research Committee, Federal University of São Paulo (process number 0896/03).
After the recruitment process was completed, all included children were referred to the Neuro-Sono sleep clinic, Department of Neurology, Federal University of São Paulo. We excluded children who had undergone surgical treatment of the oral cavity or nasopharyngeal airway space, such as tonsillectomy, adenoidectomy, or adenotonsillectomy. We also excluded children who were currently undergoing or had previously undergone orthodontic or facial orthopedic treatment.
Procedure
We evaluated anthropometric data for all children according to the 2007 World Health Organization criteria and determined the body mass index (BMI).17
To classify children as nose or mouth breathers, an otolaryngologic evaluation was carried out, including nasofibroscopy (Machida, Tokyo, Japan), looking for the presence of rhinitis or upper airway obstruction caused by hypertrophic tonsils or adenoids. We adopted the classification of Cassano et al.18 for determining adenoid hypertrophy; significant hypertrophy was considered to be present when a 75% or greater obstruction was detected in the airway through the nasofibroscopic evaluation.18 The same approach was adopted when evaluating nasal concha and tonsils. Criteria for classification of the child as a mouth breather included (1) parent report that the child breathes through the mouth, sleeps with the mouth opened, and dribbles on the pillow 3 times a week or more and (2) adenoid obstruction was identified on the nasofibroscopy examination. Children not meeting these criteria were classified as nasal breathers. Loud and continuous snoring was considered to be a secondary criterion for classification purposes because of the variable and subjective nature of the information. All children underwent an orthodontic evaluation (data not provided in this study), polysomnography, and lateral teleradiography to obtain cephalometric tracings.
Polysomnography
All overnight polysomnograms were performed at the Neuro-Sono Sleep Laboratory, UNIFESP, SP, Brazil. A Neurotec® EQSA-400 (Itajuba, MG, Brazil) was used to monitor electroencephalography (C3/A2, C4/A1, O1/A2, and O2/A1), right and left electrooculography, submental electromyography, and electrocardiography (modified D1). The arterial oxygen saturation was monitored via finger pulse oximetry (Model Pulse 504, Criticare® Systems, Inc., Waukesha, WI). Nasal pressure and oronasal flow were measured using a 3-pronged, 3-way thermistor. Chest and abdominal wall movements were measured using piezoelectric belts, and leg movements were monitored using superficial anterior tibialis muscle electrodes.
Polysomnography was scored for sleep stages according to the standard Rechtschaffen and Kales criteria19 using 30-second epochs.19,20 Respiratory events were scored according to standard criteria for children.21–23 Obstructive apnea was defined as cessation of airflow, lasting for at least 2 breaths, in the presence of paradoxical ribcage and abdominal movements. Hypopnea was defined as a reduction of the thermistor signal by more than 50% that was accompanied by either oxygen desaturation or arousal. Central apnea was defined as the absence of airflow at both the nose and mouth with absent inspiratory effort throughout the entire duration of the event, lasting 20 seconds or longer, or 2 missed breaths accompanied by at least a 3% oxygen desaturation, an arousal, or an awakening. The obstructive apnea index was defined as the number of obstructive apneas per hour of sleep. The apnea-hypopnea index (AHI) was defined as the number of obstructive apneas and hypopneas per hour of sleep.21,23,24 An AHI of 0 was considered to be normal.22 According to Marcus et al.'s criteria,22 the SaO2 was classified as normal if it remained at 92% or higher during the total sleep time and abnormal if the SaO2 nadir dropped to less than 92%.22,25
Snoring was defined as a loud breathing produced mainly by the vibration of the soft palate and oropharyngeal pillars. Based on polysomnography, snoring was determined to be absent or present in each child, and, if present, the snoring was scored as slight, moderate, or severe.
Based on the American Sleep Disorders Association criteria,20 3-second or longer arousals were classified into (1) arousals within 2 seconds of the termination of an obstructive apnea or hypopnea and (2) arousals not associated with an obstructive apnea or hypopnea (spontaneous arousals).24
Sleep latency was defined as the time from lights off to the beginning of sleep and was classified as normal (up to 20 minutes) or increased.13 Sleep efficiency was defined as the ratio of the total sleep time and the total recorded time and was classified as normal (above 89%) or decreased.13
Periodic limb movements of sleep were defined as periodic, stereotypic limb movements lasting 0.5 to 5 seconds (5 or more movements every 90 seconds)20; periodic limb movement of sleep indexes were classified as normal in subjects with fewer than 5 movements per hour of total sleep time.
Teleradiography
Lateral radiographs were obtained with the children in a seated position, with their teeth in normal occlusion. Ear rods placed on the auricular orifice allowed the Frankfurt plane to be maintained parallel to the ground. Before they underwent vertical lateral teleradiography, all of the children washed their mouths and swallowed barium sulfate, 10 mL, to allow for visualization of the soft tissue structures, such as the tongue, soft palate, and epiglottis. An EMIC X-ray model MKT 100 was used, maintaining a distance of 152 cm from the radiograph emission point to the cephalostat center.
Masking
Polysomnograms and teleradiographs were delivered directly to the responsible person in the secretarial office of the research center, who masked the identification of the subjects, renaming the files of the sleep studies and keeping the identification and the newly assigned name in a closed record. The identification of each teleradiograph was hidden by opaque tags, and each teleradiograph was randomly filed in a numbered envelope. After the population data were collected, 1 of the authors (LBFP) reported the polysomnographic results, whereas the other author (MLJ) used a negatoscope and acetate paper to trace the radiographs; both authors were blinded to the identification of the subjects.
Measurements
The anatomic design and tracings of lines and planes were performed over the radiographs (Figure 1) to determine the measured variables. We compared the cephalometric measures between groups and with normal cephalometric parameters for children (Table 1). To evaluate the intraobserver agreement between cephalometric measures, we retraced 10 radiographs, without knowledge of the measures determined by the first observer.
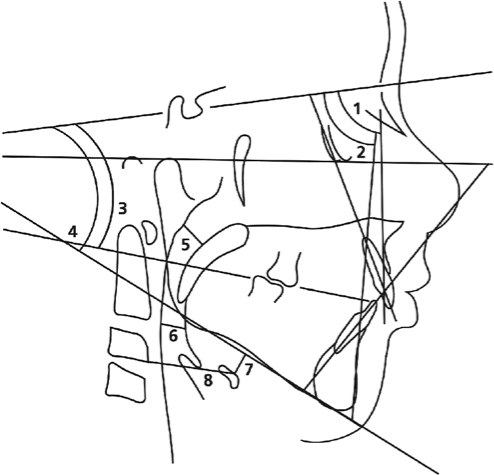
Figure 1.
Anatomic drawing, linear measurements, and angles traced for the determination of the cephalometric variables: 1, SNA; 2, SNB; 3, NSPlO; 4, NSGoGn; 5, SPAS; 6, PAS; 7, MPH; 8, C3H.
Table 1.
Normal Cephalometric Data for Children
| Cephalometric Measurements | Description | Diagnostic value | Normal value |
|---|---|---|---|
| SNA | Angle formed by the sella-nasion line and line N-point A | Anteroposterior position of the maxilla in relation to the skull base | 82° |
| SNB | Angle formed by the sella-nasion line and line N-point B | Anteroposterior position of the mandible in relation to the skull base | 80° |
| ANB | Differences between the SNA and SNB angles | The relation between maxilla and mandible | 2° |
| NS.PlO | Angle formed by the sella-nasion line and the occlusal plane | The inclination of the occlusal plane in relation to the skull base | 14° |
| NS.GoGn | Angle formed by the sella-nasion line and mandibular plane | The inclination of the mandibular plane in relation to the skull base | 36° |
| 1.NA | Angle of inclination of the upper incisor in relation to the NA line | The extent of anterior inclination of the upper incisor | 22° |
| 1-NA | Linear distance between the most salient point of the buccal side of the upper incisor and the NA line measured perpendicularly to the latter | The extent of anterior inclination of the upper incisor | 4 mm |
| 1.NB | Angle of inclination of the lower incisor in relation to the NB line, which determines the extent of anterior inclination of the lower incisor | The extent of anterior inclination of the lower incisor | 25° |
| 1-NB | Linear distance between the most salient point of the buccal side of the lower incisor and the NB line measured perpendicularly to the latter | The extent of anterior inclination of the lower incisor | 4 mm |
| SPAS | The thickness of the airway behind the soft palate along a line parallel to the Go-B point plane | Thickness of superior posterior airway space | 10 mm |
| PAS | Linear distance between a point at the base of the tongue and another point on the posterior wall of the pharynx, both measured by the extension of a line from point B to point Go13 | Thickness of posterior airway space | 10 mm |
| MP-H | Linear distance between H, the most anterosuperior point of the hyoid bone, and the mandibular plane measured perpendicularly to the latter13 | Risk of occlusion, that increases directly with the distance | 18 mm |
| C3-H | Linear distance between C3 and H, where C3 is the most anteroinferior point of the third cervical vertebra36 | Risk of occlusion, that increases inversely with the distance | 35 mm |
Statistical Analysis
The values of each variable for each patient were entered into an electronic chart (Excel, Microsoft Corp., Redmond, WA). The identity of nose breathers and mouth breathers were revealed, and the 2 groups were then constructed regardless of age or sex.
We used the χ2 and Fisher exact tests to compare polysomnographic variables between the nose-breather and mouth-breather groups, except for the analysis of arousal episodes, for which we used the Student t test. To use χ2 and Fisher exact tests to detect associations between polysomnography variables and cephalometric data, we categorized the polysomnography data as follows: AHI normal (< 1.0) or abnormal (≥ 1.0)22; SaO2 normal (SaO2 of at least 92% during the entire sleep study) and abnormal (at least 1 desaturation event below 92% during the sleep study)22,25; snoring absent (no report of loud breathing sounds, i.e., the parents or technician—while wiring [if the child was already sleeping] or manipulating sensors during the night or in the control center loudspeakers—did not perceive the child as snoring) or present (the technician reported slight, moderate, or severe snoring); sleep latency normal (≤ 20 minutes) or increased (> 20 minutes)13; sleep efficiency normal (> 89%) or decreased (< than 89%)13; and periodic limb movements of sleep normal (≤ 5 movements per hour) or abnormal (> 5 movements per hour).20 All normal and abnormal categories were supported by evidence-based literature, mostly consensus papers by the American Academy of Sleep Medicine,20,21,23 and the normative values established by the Carskadon13 and Marcus22 groups.
Student t tests and χ2 tests were used to compare cephalometric measurements of nose- and mouth-breathing children based on age, sex, and BMI. Multiple linear regression was used to evaluate the interaction of the arousal index with the remaining cephalometric measurements, and logistic regression was used to evaluate the interactions among polysomnography variables and cephalometric measurements adjusted according to group (nose-breathing and mouth-breathing). To evaluate the intraobserver agreement, we used the κ statistic, and classified agreements as follows: perfect (κ = 0.81-1.0), substantial (κ = 0.61-0.8), moderate (κ = 0.41-0.6), fair (κ = 0.21-0.4), slight (κ = 0.01-0.2), and poor (κ = 0.00). We considered a p value of less than 0.05 to be statistically significant.
In addition, the error of the method was determined by repeating the measurement in 10 random cephalometric radiographs (37% of our sample) for all variables according to the Dahlberg formula (S2 = Σd2/2n, where S refers to the random error, d is the difference between repeated radiographs recorded, and n is the number of radiographs recorded), as recommended by Houston.26 The 2 sets of measurements were obtained by retracing the radiographs and making another cephalometric measurement. Each cephalogram was traced and measured again by the same author. We also calculated the variance of error in the percentage of variance for the NSGoGn according to Midtgard et al.28
RESULTS
Of the 27 children (18 boys), 15 (9 boys) were mouth breathers and 12 (9 boys) were nose breathers. Age was similar between groups (nose breathers: 10.3 ± 1.4 years; mouth breathers: 9.5 ± 1.8 years; p = 0.11). The BMI of nose breathers was 18.0 ± 1.9 kg/m2, and of mouth breathers was 17.7 ± 2.1 kg/m2 (p = 0.37). The nose-breathing group included 2 overweight children, and the mouth-breathing group included 5 (p = 0.4). Intragroup analysis did not show any difference in the presence of an AHI of 1 or more per hour (p = 0.57) or of an SaO2 of 92% or less (p = 1.0). All children in the mouth-breathing group, as expected, snored, independent of BMI, and, for the nose-breathing group, 1 overweight child snored, and the other overweight child did not (p = 1.0). Mouth-breathing and nose-breathing groups did not differ regarding sex ratios (p = 0.68), and within-group BMIs and sex ratios were also not different (p = 1.0). (Table 2)
Table 2.
Demographic Data for the 27 Children Studied
| Parameter | Mouth breathers | Nose breathers | p Value |
|---|---|---|---|
| Sex, n | 0.68 | ||
| Boys | 9 | 9 | |
| Girls | 6 | 3 | |
| Age, y | 9.5 ± 1.8 | 10.3 ± 1.4 | 0.11 |
| BMI, kg/m2 | 17.7 ± 2.1 | 18.0 ± 1.9 | 0.37 |
| Overweight, n | 5 | 2 | 0.4 |
Data are presented as number or mean ± SD. BMI refers to body mass index.
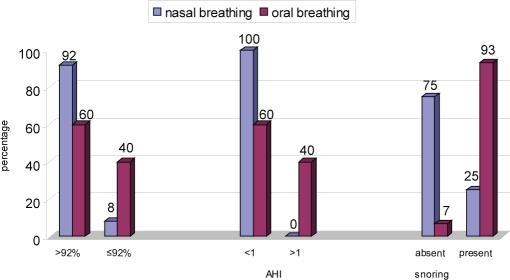
The number of children with a decreased sleep efficiency, an increased sleep latency, and a higher than normal periodic limb movements of sleep index was similar in both groups. Both groups of children had a similar number of arousals (p = 0.66). The number of children with an SaO2 desaturation was greater in the mouth-breathing group than in the nose-breathing group (p = 0.09). The mouth-breathing group had more children with an AHI greater than 1 (p = 0.02) and had more children who snored (p < 0.001), as compared with the nose-breathing group. (Figure 2)
Figure 2.
Oxygen saturation, apnea-hypopnea index (AHI), and snoring in mouth- and nose-breathing children.
The children in the mouth-breathing group were more likely to have a retruded mandible relative to the base of the cranium, according to the SNB measurement (p = 0.01), and relative to the maxilla, according to the ANB measurement (p = 0.004); they also had a more inclined occlusal plan, according to the NS.PlO measurement (p = 0.002), and a steeper mandibular plane, according to the NS.GoGn measurement (p = 0.002). The mouth-breathing group had a smaller airway space than the nose-breathing group, according to the SPAS measurements (p < 0.0001) and PAS (p = 0.02) (Table 3).
Table 3.
Cephalometric Measurements in Nose- and Mouth-Breathing Children
| Cephalometric measures | Normal value | Nose breathers | Mouth breathers | p Value |
|---|---|---|---|---|
| SNA, ° | 82 | 85.67 ± 5.26 | 83.33 ± 3.99 | 0.20 |
| SNB, ° | 80 | 80.83 ± 5.25 | 76.20 ± 4.04 | 0.01a |
| ANB, ° | 2 | 4.58 ± 1.44 | 7.07 ± 2.46 | 0.004a |
| NS.PlO, ° | 14 | 16.50 ± 5.28 | 22.00 ± 3.32 | 0.002a |
| NS.GoGn, ° | 32 | 30.25 ± 7.21 | 38.53 ± 5.63 | 0.002a |
| 1.NA, ° | 22 | 26.58 ± 4.98 | 25.13 ± 6.91 | 0.54 |
| 1-NA, mm | 4 | 5.25 ± 1.76 | 4.40 ± 2.72 | 0.35 |
| 1.NB,° | 25 | 33.25 ± 6.34 | 30.27 ± 7.57 | 0.28 |
| 1-NB, mm | 4 | 6.08 ± 1.62 | 6.87 ± 2.53 | 0.36 |
| SPAS, mm | 10 | 1.25 ± 3.47 | 4.47 ± 1.68 | < 0.0001a |
| PAS, mm | 10 | 12.58 ± 2.97 | 9.93 ± 2.84 | 0.02a |
| MP-H, mm | 18 | 11.58 ± 7.25 | 14.40 ± 5.17 | 0.24 |
| C3-H, mm | 35 | 34.33 ± 3.94 | 32.27 ± 2.60 | 0.11 |
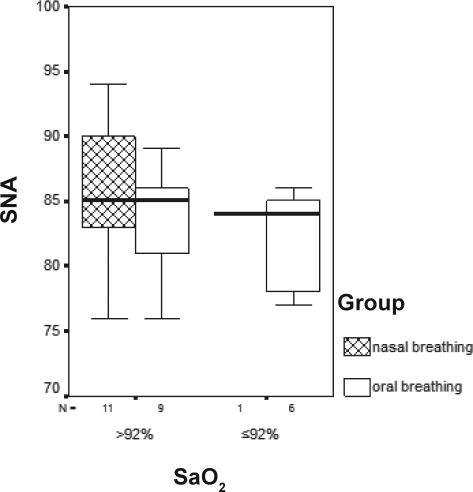
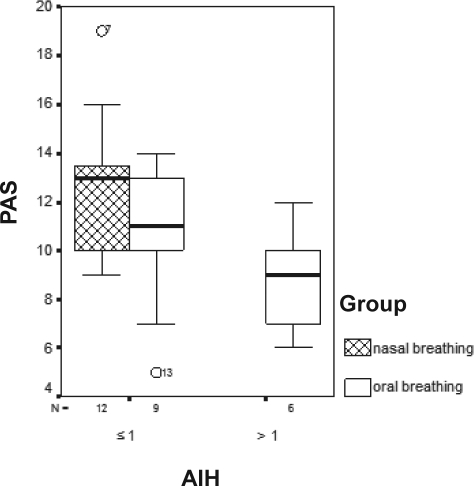
Snoring mouth breathers had smaller SPAS (p = 0.005), as compared with nose breathers (Figure 3). Mouth breathers who had oxygen desaturations had smaller SNA measurements (p = 0.09) (Figure 4), and those with an AHI greater than 1 had smaller PAS measurements (p = 0.05), when compared with the nose breathers (Figure 5).
Figure 3.
Values of SPAS by presence or absence of snoring for mouth- and nose-breathing children. p = 0.005.
Figure 4.
Values of SNA by oxygen saturation (SaO2) for mouth- and nose-breathing children. p = 0.09.
Figure 5.
Values of PAS by apnea-hypopnea index (AHI) for mouth- and nose-breathing children.
The chance of a child snoring increased 1.61 times with every 1-mm decrease in SPAS. That is, children who snored had a decreased SPAS, and the odds of a snoring child being a mouth breather was 3.73 times higher than the odds of being a nose breather (p = 0.002).
The multiple linear regression models showed that SPAS measurement and snoring were associated (p = 0.0053). There was a trend toward oxygen desaturation with the decrease in SNA measurement (p = 0.09). AHI increased when there was a decrease in the PAS (p = 0.05).
For the measurement agreement, our data can be considered reliable. Four measures (NSGoGn, 1-NB, SPAS, MPH) showed perfect agreement, 5 measures (NSPlO, 1.NA, 1.NB, PAS, C3H) showed substantial agreement, and 3 measures (SNA, SNB, ANB) showed moderate agreement. Only 1 measure (1-NA) showed fair agreement (κ = 0.21 to 0.4), but this measure did not show a significant association with any variables in our study. Measurement errors estimated according to the Dahlberg formula ranged from 0.8 to 0.9 mm for linear measurements and 0.5° to 1.4° for angle measurements (Table 4). Error was marginally significant (p = 0.051) only for NSGoGn. Variance of error in the percentage of variance in the material as a whole was 3.4% according to the Midtgard approach.28
Table 4.
Intrascorer Reliability of Repeated Linear and Angle Measurement by the Dahlberg Method
| Variable | First measure | Second measure | p Value | SE |
|---|---|---|---|---|
| SNA,° | 82.7 ± 2.71 | 82.8 ± 3.25 | 0.79 | 0.8 |
| SNB,° | 77.6 ± 2.95 | 77.8 ± 3.32 | 0.44 | 0.5 |
| ANB,° | 5.1 ± 1.85 | 5.0 ± 1.88 | 0.67 | 0.5 |
| NSPlO,° | 18.4 ± 3.83 | 19.3 ± 3.94 | 0.12 | 1.2 |
| NSGoGn,° | 36 ± 9.35 | 37.2 ± 8.28 | 0.05 | 1.4 |
| SPAS, mm | 8.5 ± 5.01 | 8.5 ± 5.81 | 1.00 | 0.8 |
| PAS, mm | 12.1 ± 2.13 | 12.7 ± 1.88 | 0.16 | 0.9 |
Data are presented as mean ± SD. SE refers to standard error.
DISCUSSION
This study showed that, compared with nose-breathing children, mouth-breathing children had more oxygen desaturations during sleep, had higher AHI levels, and snored more; the results also indicated that each 1-mm decrease in the SPAS increased the odds of snoring 1.61 times. Mouth breathers had abnormal maxillary-mandible ratios, with mandibular retrusion relative to the base of the cranium and increased anterior facial height (as seen by an increase in the NS.GoGn angle and an increase in the occlusal plane inclination angle). The cephalometry of mouth breathers also showed a smaller upper airway space with a clearly narrowed area at the level of the nasopharynx, hypopharynx, or both.
The study included children 7 to 14 years of age (mean age of 10 ± 1.1 years). We know that, at 12 years of age, the craniofacial skeleton has reached 90% of its growth.8 Moreover, the children in this study were not subject to orthodontic or facial orthopedic treatment, and, thus, the craniofacial changes observed in cephalometry will remain in adulthood unless the child's craniofacial growth is changed by means of facial orthopedic treatment.
Craniofacial abnormalities were more frequent in mouth breathers than in nose breathers, which is in agreement with the results of several studies comparing both groups of children.15,29,30 Caprioglio et al.30 and Kulnis et al.31 used cephalometric radiography to compare craniofacial parameters of children who habitually snored and children who did not snore; they concluded that children who snored had smaller SPAS and PAS measurements than did nonsnoring children. The frequency of snoring increases progressively in mouth-breathing children due to factors such as allergic rhinitis, adenoid hypertrophy, and adenotonsillar hypertrophy,32 and, according to Nishimura and Suzuki's33 data, snoring and OSAS are closely associated with morphologic changes caused by mouth breathing. Interestingly, our findings showed smaller SPAS and PAS measurements in mouth breathers, compared with nasal breathers; the same finding was also observed in snoring versus nonsnoring children. Between-study comparisons may be difficult because researchers may use different criteria (snoring vs nonsnoring children or mouth vs nose breathers) or because the children may have the same disease with 2 different (but related) clinical expressions (snoring and mouth breathing). This association between snoring and mouth breathing may not be obvious because, even in our small sample of 12 nose breathers, we had 3 children who snored.
It is well known that snoring is a predictor of SDB,2 and, in our study, SPAS measurement had the highest correlation with snoring; thus, when orthodontists find a decreased SPAS, they should determine whether the child is a habitual snorer or has a more severe sleep-related breathing disorder such as OSAS.
Children with OSAS often have growth deficits,34 cognitive problems,35 delayed learning,36 social disability,37 and behavior disorders.38 Snoring is a noisy event during sleep caused by the vibration of the tissues obstructing the nasopharynx and oropharinx.39 This obstruction is enough to cause oxygen desaturation, which compromises adequate oxygenation of the tissues and brain in children, explaining the poor cognitive performance.36 Recent studies have shown that, even if the child does not have apnea confirmed by polysomnography, the presence of snoring alone is enough to explain the symptoms described above.36,40 At night, the major characteristic of children with SDB is agitated sleep, snoring, and breathing difficulties, which may start early in life. The consequences for the growth and development of these children are dramatic, and, therefore, the early diagnosis and treatment SDB are extremely important.
The association between SDB and mouth breathing has been well established.30,41,42 Children with habitual snoring have craniofacial modifications that contribute to a posterior crossbite caused by a change in maxillary growth after continuous mouth breathing and an anterior open bite with lip incompetence due to the anterior positioning of the tongue.15 Our data show that mouth-breathing children also have an increased anterior facial height, observed by the measurement of NS.GoGn angle and by a greater inclination of the occlusal plane that is related to an open bite and lip incompetence. These data are in agreement with those of other authors who have studied children with SDB.43,44 Mouth breathers also have a retruded mandible relative to the base of the cranium and the maxilla, shown by SNB and ANB measurements, respectively, favoring the occurrence of a small oral cavity and retropositioning of the tongue,15,28 which are anatomic aspects usually found in patients with SDB.15,27–29
Children who have an abnormality in dental occlusion or positioning of the teeth usually search for an orthodontist to provide orthodontic or facial orthopedic repair. Therefore, it is extremely important for orthodontists to pay close attention to the information and interpretation of cephalometric measurements, since they may identify children who have SDB that has not previously been diagnosed by a health professional even though the professional may have examined the child. According to our study results, children who have a decreased SPAS measurement, confirmed by cephalometry, are highly likely to snore.
The overall intraobserver agreement of our measurement approach was remarkably good; the κ values were at least 0.6. There was good agreement between categoric measurements when the same author retraced the cephalograms to obtain angle and linear measures on different days (κ > 0.6). Only 1-NA showed fair agreement, but this measurement was not statistically significant. Random error assessed by the Dahlberg formula also ensured a good reproducibility, and only NSGoGn showed a marginally significant error. This same measurement was perfectly in agreement with κ statistics, and, most importantly, this measurement variation did not have a clinical impact on the cephalometric evaluation, ie, a patient in a specified class (normal or abnormal) remained in that class even though a range of 0.5° to 1.4° was observed. Another important point in this issue is that the variance error in the percentage of variance was 3.4% for NSGoGn, which is pretty close to the rigorous 3% proposed by Midtgard et al.28 Those authors considered that the variance of error should not exceed 3%, and, if the variance of error did exceed 10% of the variance in the material as whole for that specified landmark, then the applied method of measuring is inappropriate.
The most important limitation of our study is the small number of children studied. After we analyzed the data, we noticed that only 1 child was in the nonsnoring mouth-breather group, 3 in the snoring nose-breather group, and only 3 girls who were nose breathers. Although such data could be identified only after polysomnography results were revealed, we think that it would be of interest to study a larger number of nonsnoring mouth-breathing children and snoring nose-breathing children selected after polysomnography was conducted. Studying predominantly boys may also be of concern, but, in this study, our aim was to see if any cephalometric variables were associated with polysomnographic data. Therefore, the predominance of boys does not invalidate our findings.
In conclusion, this study showed that teleradiography may be an auxiliary tool in children to predict SDB diagnosed by polysomnography, and the correct interpretation of cephalometric data may result in an early diagnosis of SDB. Snoring was the most important variable associated with abnormal craniofacial morphology. Reduced pharyngeal airway space, significant maxillary protrusion, or a retruded mandible (overjet) and a steeper mandibular plane should be considered as potentially predictive of respiratory polysomnographic findings. Because every orthodontic treatment requires cephalometric evaluation, we encourage orthodontists to be aware of SDB and to refer children with abnormal findings to a sleep specialist.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Marcus CL. Sleep-disordered breathing in children. Am J Respir Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JL. Sleep-related upper-airway obstruction in children and adolescents. Child Adolesc Psychiatry Clin North Am. 1996;5:617–47. [Google Scholar]
- 3.Marcus CL. Obstructive sleep apnea syndrome: differences between children and adults. Sleep. 2000;23:S140–1. [PubMed] [Google Scholar]
- 4.Warren DW. Effect of airway obstruction upon facial growth. Otolaryngol Clin North Am. 1990;23:699–712. [PubMed] [Google Scholar]
- 5.Sousa JBR, Anselmo-Lima WT, Valera FCP, Gallego AJ, Matsumoto MAN. Cephalometric assessment of mandibular growth pattern in mouth-breathing children. Int J Pediatr Otorhinolaryngol. 2005;69:311–7. doi: 10.1016/j.ijporl.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Oulis CJ, Vadiakas GP, Ekonomides J, Dratsa J. The effect of hypertrophic adenoids and tonsils on the development of posterior crossbite and oral habits. J Clin Pediatr Dent. 1994;18:197–201. [PubMed] [Google Scholar]
- 7.Kerr WJS, Orth D, McWilliam JS, Linder-Aronson S. Mandibular form and position related to changed mode of breathing – a five-year longitudinal study. Angle Orthod. 1989;59:91–6. doi: 10.1043/0003-3219(1989)059<0091:MFAPRT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Woodside DG, Linder-Aronson S, Lundstrom A, McWilliam J. Mandibular and maxillary growth after change mode of breathing. Am J Orthod Dentofacial Orthop. 1991;100:1–18. doi: 10.1016/0889-5406(91)70044-W. [DOI] [PubMed] [Google Scholar]
- 9.Cheng MC, Enlow DH, Papsidero M, Broadbent BH, Jr, Oyen O, Sabat M. Developmental effects of impaired breathing in the face of growing child. Angle Orthod. 1988;58:309–20. doi: 10.1043/0003-3219(1988)058<0309:DEOIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Mehra P, Downie M, Pita MC, Wolford LM. Pharyngeal airway space changes after counterclockwise rotation of the maxillomandibular complex. Am J Orthod Dentofacial Orthop. 2001;120:154–9. doi: 10.1067/mod.2001.114647. [DOI] [PubMed] [Google Scholar]
- 11.Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90:484–91. doi: 10.1016/0889-5406(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 12.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rochette HE., Jr Craniofacial structure and obstructive sleep apnea syndrome - a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996;109:163–72. doi: 10.1016/s0889-5406(96)70177-4. [DOI] [PubMed] [Google Scholar]
- 13.Acebo C, Millman RP, Rosenberg C, Cavallo A, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Part I—normative values. Chest. 1996;109:664–72. doi: 10.1378/chest.109.3.664. [DOI] [PubMed] [Google Scholar]
- 14.Millman RP, Acebo C, Rosenberg C, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Part II—response to nasal occlusion. Chest. 1996;109:673–9. doi: 10.1378/chest.109.3.673. [DOI] [PubMed] [Google Scholar]
- 15.Zucconi M, Caprioglio A, Calori G, et al. Craniofacial modifications in children with habitual snoring and obstructive sleep apnoea: a case-control study. Eur Respir J. 1999;13:411–7. doi: 10.1183/09031936.99.13241199. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Chest. 1984;86:793–4. doi: 10.1378/chest.86.5.793. [DOI] [PubMed] [Google Scholar]
- 17.Onis M, Onyango AW, Borghi E, Siyan A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassano P, Gelardi M, Cassano M, Fiorella ML, Fiorella R. Adenoid tissue therapeutic management. Int J Pediatr Otorhinolaryngol. 2003;67:1303–9. doi: 10.1016/j.ijporl.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/UCLA; 1968. [Google Scholar]
- 20.The American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 22.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Wong TK, Galster P, Lau TS, Lutz JM, Marcus CL. Reliability of scoring arousals in normal children and children with obstructive sleep apnea syndrome. Sleep. 2004;27:1139–45. doi: 10.1093/sleep/27.6.1139. [DOI] [PubMed] [Google Scholar]
- 25.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 26.Bibby RE, Preston CB. The hyoid triangle. Am J Orthod. 1981;80:92–7. doi: 10.1016/0002-9416(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 27.Houston WJB. The analysis errors in orthodontic measurements. Am J Orthod. 1983;83:382–90. doi: 10.1016/0002-9416(83)90322-6. [DOI] [PubMed] [Google Scholar]
- 28.Midtgard J, Björk G, Linder-Aronson S. Reproducibility of cephalometric landmarks and errors of measurement of cephalometric cranial distances. Angle Orthod. 1974;44:56–61. doi: 10.1043/0003-3219(1974)044<0056:ROCLAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein Y, Wexler D, Berger G, Nachmany A, Shapiro-Feinberg M, Ophir D. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. Arch Otolaryngol Head Neck Surg. 2000;126:593–600. doi: 10.1001/archotol.126.5.593. [DOI] [PubMed] [Google Scholar]
- 30.Caprioglio A, Zucconi M, Calori G, Troiani V. Habitual snoring, OSA and craniofacial modification. Orthodontic and diagnostic aspects in a case control study. Minerva Stomatol. 1999;48:125–37. [PubMed] [Google Scholar]
- 31.Kulnis R, Nelson S, Strohl K, Hans M. Cephalometric assessment of snoring and nonsnoring children. Chest. 2000;118:596–603. doi: 10.1378/chest.118.3.596. [DOI] [PubMed] [Google Scholar]
- 32.Di Francesco RC, Passerotii G, Paulucci B, Miniti A. Mouth breathing in children: different repercussions according to the diagnosis. Rev Bras Otorrinolaringol. 2004;70:665–70. [Google Scholar]
- 33.Nishimura T, Suzuki K. Anatomy of oral respiration: morphology of oral cavity and pharynx. Acta Otolaryngol. 2003;550(Suppl):25–8. doi: 10.1080/0365523031000061. [DOI] [PubMed] [Google Scholar]
- 34.Marcus CL, Carroll JL, Koerner CB, Hamer A, Lutz J, Loughlin GM. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr. 1994;125:556–62. doi: 10.1016/s0022-3476(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho LB, Prado LF, Silva L, et al. Cognitive dysfunction in children with sleep-disordered breathing. J Child Neurol. 2005;20:400–4. doi: 10.1177/08830738050200050101. [DOI] [PubMed] [Google Scholar]
- 36.Gozal D, Pope DW., Jr. Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107:1394–9. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- 37.Crabtree VM, Vami J, Gozal D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27:1131–8. doi: 10.1093/sleep/27.6.1131. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13:165–72. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 39.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a development perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 40.Gozal D, O'Brien L, Row BW. Consequences of snoring and sleep disordered breathing in children. Pediatr Pulmonol. 2004;Sup26:166–8. doi: 10.1002/ppul.70094. [DOI] [PubMed] [Google Scholar]
- 41.Peltomäki T. The effect of mode of breathing on craniofacial growth-revisited. Eur J Orthod. 2007;29:426–9. doi: 10.1093/ejo/cjm055. [DOI] [PubMed] [Google Scholar]
- 42.McLean HA, Urton AM, Driver HS, et al. Effect of treating severe nasal obstruction on the severity of sleep apnoea. Eur Resp J. 2005;25:521–7. doi: 10.1183/09031936.05.00045004. [DOI] [PubMed] [Google Scholar]
- 43.Bacon WH, Turlot JC, Krieger J, Stierle JL. Cephalometric evaluation of pharyngeal obstructive factors in patients with sleep apnea syndrome. Angle Orthod. 1990;60:115–22. doi: 10.1043/0003-3219(1990)060<0115:CEOPOF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Kawashima S, Niikuni N, Chia-hung L, et al. Cephalometric comparisons of craniofacial and upper airway structures in young children with obstructive sleep apnea syndrome. Ear Nose Throat J. 2000;79:499–506. [PubMed] [Google Scholar]