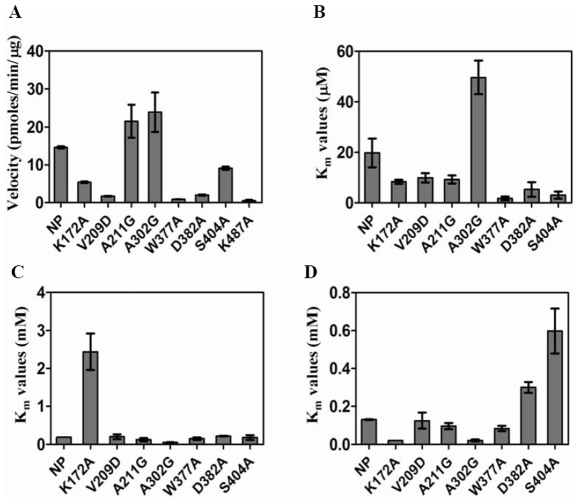
Figure 6. Comparison of the kinetic properties of FadD13 and its mutants.
Km values for substrates and Vmax values were determined by performing FadD13 assays as described in the section on “Materials and Methods”. For the calculation of Michaelis constants, a varying range of substrate concentrations (Palmitic acid – 1.5–60 µM, ATP - 0.03–4 mM, CoenzymeA - 0.02–1.5 mM) were employed and the Km values were calculated by GraphPad Prism 5 (San Diego, California, USA) by using a non-linear fitting method. (A) Comparison of Vmax values of the native FadD13 (NP) and its mutants. Km values of Palmitic acid (B), ATP (C) and CoenzymeA (D) for native FadD13 (NP) and its mutants. The data is depicted as mean of values ± S.E. of two independent experiments carried out in duplicates.