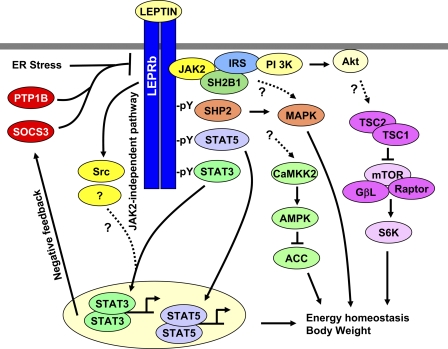
Fig. 2.
A model of leptin signaling and leptin resistance. Leptin binds to the long form of the leptin receptor (LEPRb) and activates LEPRb-associated JAK2. JAK2 phosphorylates LEPRb on Tyr985/1077/1138. SH2-containing protein tyrosine phosphatase 2 (SHP2) binds to phospho-Tyr985 and mediates the activation of the MAPK pathway. Suppressor of cytokine signaling-3 (SOCS3) also binds to phospho-Tyr985 and inhibits leptin signaling in a negative feedback manner. STAT5 and STAT3 bind to phospho-Tyr1077 and phospho-Tyr1138, respectively, and are subsequently phosphorylated and activated by JAK2. STAT3 and STAT5 activate their target genes, which mediate leptin's anorexigenic effect. JAK2 autophosphorylates on Tyr813, which binds to SH2B1. SH2B1 simultaneously binds to insulin receptor substrate (IRS)-1 and IRS-2 and recruits IRS proteins to the LEPRb/JAK2 complex, which results in JAK2-mediated tyrosine phosphorylation of IRS-1 and IRS-2 and subsequent activation of the phosphoinositide 3-kinase (PI3K) pathway. Leptin also stimulates a JAK2-independent pathway involving the Src tyrosine kinase family members. The JAK2-dependent and -independent pathways act coordinately and synergistically to promote STAT3 activation. Leptin also regulates the CaMKK2/AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) and the mammalian target of rapamycin (mTOR)/ribosomal S6 kinase (S6K) pathways; however, the molecular steps from the LEPRb to these 2 pathways are not clear. These diverse pathways act coordinately as a network to fully mediate leptin responses. LEPRb signaling is negatively regulated by SOCS3, protein tyrosine phosphatase 1B (PTP1B), and endoplasmic reticulum (ER) stress but positively regulated by SH2B1.