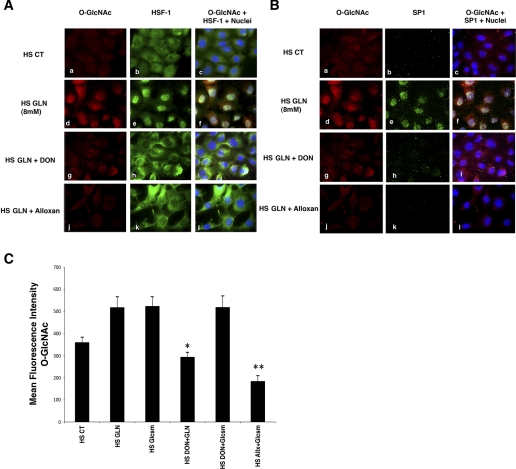
Fig. 6.
Accumulation of nuclear O-GlcNAc modified HSF-1 and Sp1 after GLN treatment in mouse embryonic fibroblast (MEF) cells following heat stress. Cells treated with 0 or 8 mM GLN underwent heat stress as described in experimental procedures. Cy3-labeled O-GlcNAc (shown in red), Alexa Fluor 488-labeled HSF-1 (green; A) and Cy5-labeled SP1 (green; B) were visualized for cellular localization and levels. A: HSF-1 nuclear accumulation. Control cells show minimal O-GlcNAc staining (top, a) and predominantly cytoplasmic HSF-1 (top, b) following heat stress. GLN-treated cells show an increase in cellular O-GlcNAc-modified proteins (middle, d) and increased nuclear translocation of the transcription factor HSF-1 (middle, e). Nuclear colocalization of HSF-1 and O-GlcNAc residues appear yellow (middle, c, f, i, l). Both DON and alloxan, inhibitors of key enzymes in the HBP, attenuate GLN-mediated increase in O-GlcNAc-modified protein (middle, g and j) and nuclear HSF-1 translocation (middle, h and k). B: Sp1 nuclear accumulation. Control cells show minimal O-GlcNAc staining (top, a) and minimal Sp1 staining (top, b) following heat stress. GLN-treated cells show an increase in cellular O-GlcNAc-modified proteins (middle, d) and increased nuclear Sp1 (middle, e). Sp1 and O-GlcNAc appear to colocalize in the nucleus and again appear yellow (middle, c, f, i, l). Both DON and alloxan, inhibitors of key enzymes in the HBP, attenuate GLN-mediated increase in O-GlcNAc-modified protein (middle, g and j) (as above) and nuclear Sp1 content (middle, h and k). C: glucosamine (Glcsm) restores HBP activity in cells treated with DON. Microscopy showed that glucosamine (which activates HBP downstream of GFAT) enhanced intracellular O-GlcNAc levels as well as GLN (and also in the presence of the GFAT inhibitor, DON). DON decreased GLN-mediated O-glycosylation (*P < 0.02 vs. HS GLN), but not glucosamine-mediated O-glycosylation. Alloxan (an inhibitor of OGT) decreased both GLN and glucosamine mediated enhancement of the pathway (**P < 0.05 vs. HS glucosamine).