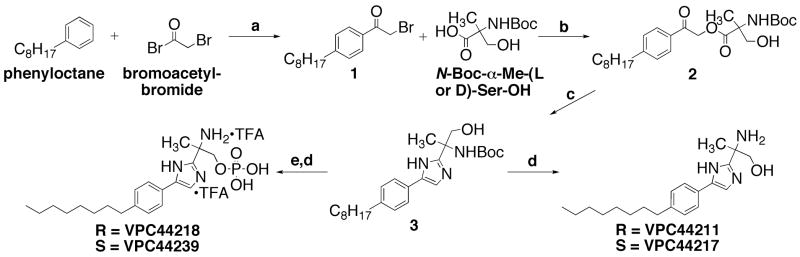
Scheme 1.
Synthesis of chiral 4(5)-phenylimidazoles. Reagents and conditions: a.) AlCl3, neat, 0 °C to rt, 4h. (84%); b.) Cs2CO3, EtOH, sonication, 5 – 10 min.; then α-bromoketone in DMF, rt, overnight (86–94%); c.) NH4OAc, Xylenes, Dean-Stark, 110–120 °C, 1–3h. (50–60%); d.) TFA, CH2Cl2, 0 °C to rt, 4h. (80–84%); e.) N,N-di-iso-propyl-di-tert-butyl-phosphoramidite, 3% tetrazole in acetonitrile, CH2Cl2, 4–8h.; then 30% H2O2(aq) rt, 4h. (33–37%).