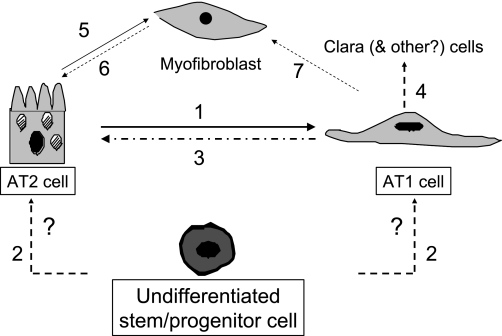
absent identification to date of less- differentiated multipotent progenitor cells within distal lung epithelium, which can give rise to either or both alveolar epithelial type II (AT2) and type I (AT1) cells, the accepted paradigm is that AT2 cells serve as progenitors for normal alveolar epithelial maintenance and repair following injury (1, 8). This concept is based on early studies of thymidine incorporation and serial morphological assessment following oxidant lung injury, which demonstrated early appearance of label in AT2 cells followed by later appearance in AT1 cells, leading to the conclusions that 1) AT2 cells are capable of self-renewal as well as differentiation into AT1 cells and 2) AT1 cells do not proliferate and are likely terminally differentiated. A limitation of these studies is that only cells that were proliferating were labeled, thereby not ruling out the possibilities of AT1 to AT2 cell transdifferentiation, transition between either cell type and less differentiated progenitors, and/or acquisition of a (myo)fibroblast phenotype through epithelial-mesenchymal transition without an intervening cell division (Fig. 1).
Fig. 1.
Alveolar epithelial cell plasticity in lung repair. The currently accepted paradigm is that, following injury, alveolar epithelial type II (AT2) cells proliferate and both self-renew and serve as precursors for replacement of AT1 cells (1). A less-differentiated bone marrow or lung-derived stem/progenitor cell in the adult that can give rise to either or both cell types has not been identified to date (2). AT1 cells in primary culture reacquire phenotypic characteristics of AT2 cells and may be induced to proliferate under appropriate experimental conditions (3). AT1 cells in vitro can be induced to express CC10, a marker of Clara cells (4). AT2 cells have been shown to undergo epithelial-mesenchymal transition to give rise to fibroblasts/myofibroblasts in vitro and in vivo (5). Reversibility of this process (mesenchymal-epithelial transition) (6) and/or if AT1 cells can transition to myofibroblasts (7) remain to be determined.
Similar to this paradigm in vivo, AT2 cells in primary culture in vitro transdifferentiate over time to an AT1 cell-like phenotype (6, 7). However, in marked contrast to the earlier in vivo results, these AT1-like cells can, under certain conditions, reacquire AT2 cell-like features, indicating that AT1 cells in vitro can exhibit phenotypic plasticity (2, 3, 4, 5). For example, culture of AT1-like cells with keratinocyte growth factor (KGF) or on contracted collagen gels reveals reexpression of surfactant proteins, loss of expression of AT1 cell markers, and reacquisition of morphological features of AT2 cells (2, 4, 5). Furthermore, although AT1-like cells manifest little proliferation at baseline, exposure to growth factors such as KGF induces significant increases in cell number (4).
These early in vitro studies demonstrating alveolar epithelial cell (AEC) phenotypic plasticity are now supported by additional in vitro data in a recent article in which Gonzalez et al. (9a) demonstrate that freshly isolated AT1 cells can proliferate and express phenotypic markers characteristic of other cell types under appropriate culture conditions. AT1 cells grown on fibronectin in the presence of 20% fetal bovine serum without added growth factors (conditions that did not support AT2 cell proliferation) exhibited a sixfold increase in cell number before becoming contact inhibited at confluence. Approximately one-half of the cells expressed the proliferation marker Ki67, consistent with proliferative capacity. AT1 cells cultured on Matrigel expressed surfactant protein C and CC10, markers of the AT2 and Clara cell phenotypes, respectively, supporting the possibility of phenotypic plasticity in vitro. When plated at very low density, ∼50% of AT1 cells formed colonies. Together with the observation that AT1 cells may express octamer-binding transcription factor 4 (Oct-4), a marker of pluripotency in embryonic stem (ES) cells, these findings led Gonzalez et al. to suggest, in addition to phenotypic plasticity and proliferative capacity, a possible progenitor function for AT1 cells.
This interesting report from Gonzalez et al. (9a) provides potentially important additional insights into AEC biology that, if recapitulated in vivo, will help alter currently accepted paradigms of normal maintenance and response to injury of alveolar epithelium. As such, interpretation and extension of these findings calls for careful consideration. For example, although Gonzalez et al. suggest that AT1 cells are capable of proliferation under the culture conditions used, expansion of the number of AT1 cells was not observed until after day 4 in culture. While perhaps unlikely given the purity of the starting populations as well as results of clonal analysis and Ki67 labeling, this delay is consistent with the possibility that, under these specific experimental conditions, a subpopulation of either less-differentiated precursor cells or RTI40-expressing cells with proliferative capacity expand after several days in culture. Serial examination of cells in culture to confirm that the early Ki67-positive cells continue to express AT1 cell markers (e.g., RTI40 and/or AQP5) will help address that possibility. Confirmation of AT1 cell plasticity in vitro also requires further evaluation of phenotype, including morphology and plasticity in clonally expanded populations.
A major limiting factor to identification of stem/progenitor cell population(s) within the alveolar epithelium has been the lack of surface markers to identify a stem cell phenotype or assays to evaluate “stemness” such as exist in the hematopoietic system (19). Bronchoalveolar stem cells, which exhibit coexpression of AT2 cell and Clara cell markers, have been suggested as possible progenitors for both proximal and distal lung epithelial cells (10). However, recent studies suggest a limited role, if any, for these cells as progenitors of distal epithelium (15, 17), and the most likely paradigm remains that AT2 cells serve as primary progenitors for maintenance and repair of the distal lung epithelium. In the current study, Gonzalez et al. (9a) demonstrate that isolated AT1 cells express Oct-4, leading to the suggestion of progenitor function for AT1 cells. Oct-4, a member of the POU family of transcription factors, represents a nuclear protein that has been suggested to function as a regulator of pluripotency in ES cells (14). The Oct-4 gene encodes two alternatively spliced isoforms (Oct-4A and Oct-4B) that differ in their NH2-terminal regions, and it is the Oct-4A isoform that is responsible for its function. However, several recent publications have suggested caution in interpretation of results of Oct-4 expression in somatic stem cells with regard to inferring pluripotency. First, accurate detection of Oct-4 is technically challenging, even with use of complementary techniques (13). Several Oct-4 retropseudogenes have been described that can confound interpretation of RT-PCR results, and it has been suggested that the most appropriate way to confirm expression of Oct-4 is to sequence the amplified PCR product to exclude false positives based on detection of a pseudogene. Evaluation of downstream targets of Oct-4 (e.g., fibroblast growth factor-4 and the transcription factor Rex-1) might also support the presence of active Oct-4 rather than a pseudogene. Western blotting may be similarly difficult to interpret, since discrimination of Oct-4A and Oct-4B isoforms based on size may be difficult. Inclusion of a competing peptide, as well as use of isotype controls, has been suggested for definitive identification of the active Oct-4A isoform. By immunostaining, only the nuclear form of Oct-4 is regarded as specific for Oct-4A, and it has been shown that the intracellular distribution of Oct-4 differs depending on the antibody used, raising questions about antibody specificity (21). Furthermore, although a number of recent studies have detected Oct-4 in somatic stem cells (particularly in vitro), questions remain regarding functional relevance of these findings in vivo and specificity of Oct-4 as an adequate marker of pluripotency in vitro (12). In this regard, conditional deletion of Oct-4 in somatic tissues of adult mice did not affect their regenerative capacity following injury, suggesting that Oct-4 is not essential for somatic stem/progenitor cell function (11). Last, demonstration of Oct-4 in adult human differentiated cells has further challenged its role as a stem cell marker, leading to the suggestion that presence of Oct-4 alone is insufficient evidence for pluripotency (20). Gonzalez et al. (9a) address some of these confounding issues by designing RT-PCR primers specific for Oct-4A and performing concurrent evaluations using Western blotting and immunofluorescence. However, potential pseudogenes were not excluded, immunostaining was predominantly but not exclusively nuclear, and identification of downstream targets of Oct-4 was not evaluated. While the data presented by Gonzalez et al. are clearly provocative, additional functional studies and evaluation of other stem cell markers in vitro and in vivo will be required to specifically attribute stem/progenitor capacity to AT1 cells.
Definitive determination that AT1 cells can give rise to AT2 or other cell types will require lineage tracing experiments in vivo in which AT1 cells are permanently labeled using Cre-lox approaches (16). This approach has facilitated analysis in tracheal/bronchial epithelium and allowed insights into the role(s) of certain cell types as progenitors (17). Approaches to lineage tagging of AT2 cells have been available for some time, but lack of appropriate promoters has delayed lineage tagging of AT1 cells. Recent characterization of several promoters unique to AT1 cells in the alveolar epithelium should permit advances in tracing AT1 cell fate and potential progenitor cell identification (9, 18). Notwithstanding these limitations, the studies by Gonzalez et al. (9a) reinforce challenges (5) to the long-held paradigm of AT1 cells as a terminally differentiated cell type (1, 8) and suggest the need for further investigation of the proliferative, plasticity, progenitor, and lineage characteristics of AT1 cells. If validated in vivo, these findings could have transformative implications for our understanding of maintenance of the alveolar epithelium and its repair following injury.
DISCLOSURES
No conflicts of interest are declared by the author(s).
GRANTS
This work was supported in part by the Hastings Foundation, Whittier Foundation, and National Heart, Lung, and Blood Institute Grants HL-038578, HL-038621, HL-062569, HL-064365, and HL-089445.
ACKNOWLEDGMENTS
E. D. Crandall is Hastings Professor and Kenneth T. Norris Jr. Chair of Medicine. Z. Borok is Ralph Edgington Chair in Medicine.
REFERENCES
- 1.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35– 42, 1974 [PubMed] [Google Scholar]
- 2.Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 275: L155– L164, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol 12: 50– 55, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 18: 554– 561, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 12: 497– 502, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Danto SI, Zabski SM, Crandall ED. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol 6: 296– 306, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 970: 146– 156, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142– 150, 1975 [DOI] [PubMed] [Google Scholar]
- 9.Flodby P, Borok Z, Banfalvi A, Zhou B, Gao D, Minoo P, Morrisey EE, Crandall ED. Directed expression of Cre in alveolar epithelial type 1 cells. Am J Respir Cell Mol Biol . In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol ( August28, 2009). doi: 10.1152/ajplung.90389.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823– 835, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1: 403– 415, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle 7: 725– 728, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Liedtke S, Stephan M, Kogler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem 389: 845– 850, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379– 391, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai L, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol 294: L1158– L1165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482– 10487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4: 525– 534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderbilt JN, Allen L, Gonzalez RF, Tigue Z, Edmondson J, Ansaldi D, Gillespie AM, Dobbs LG. Directed expression of transgenes to alveolar type I cells in the mouse. Am J Respir Cell Mol Biol 39: 253– 262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 3: 193– 207, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Zangrossi S, Marabese M, Broggini M, Giordano R, D'Erasmo M, Montelatici E, Intini D, Neri A, Pesce M, Rebulla P, Lazzari L. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells 25: 1675– 1680, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Zuk PA. The intracellular distribution of the ES cell totipotent markers OCT4 and Sox2 in adult stem cells differs dramatically according to commercial antibody used. J Cell Biochem 106: 867– 877, 2009 [DOI] [PubMed] [Google Scholar]