Abstract
Carotid body chemoreceptors transduce a decrease in arterial oxygen tension into increased action potential (AP) activity on the sinus nerve, which increases the drive to breathe. The mechanism by which AP activity increases is unresolved, but acetylcholine (ACh), acting through nicotinic receptors, is postulated to be a major contributor to nerve excitation based partly on the demonstration that pharmacological antagonism of nicotinic receptors reduces the afferent nerve response in some studies. However, most previous studies relied on indirect measures of chemoreceptor activity or utilized a recording configuration that is sensitive to AP morphology in addition to AP frequency. In the present study, single-unit AP activity was recorded from the soma of rat chemoreceptor neurons in vitro. The nicotinic blocker mecamylamine (50 μM) ablated the excitatory actions of exogenous ACh and increased, rather than decreased, AP activity during moderate hypoxia. At higher dosage (500 μM) AP height was reduced, conduction velocity slowed, and conduction failure occurred, especially during hypoxia, producing the appearance of a decreased response to hypoxia. Recovery from mecamylamine block was slow (>10 min). In contrast to mecamylamine, suramin, a P2X receptor blocker, reversibly inhibited the response to hypoxia, suggesting relatively free diffusion of drugs to the glomus cell/nerve synaptic site. These results strongly suggest that ACh acting through nicotinic receptors does not mediate excitatory transmission in rat carotid body and that previous results demonstrating such a role may have been partially influenced by changes in AP morphology or conduction failure.
Keywords: chemoreceptors, hypoxia, mecamylamine
carotid body chemoreceptors are the primary sensory organs of the respiratory system for rapid detection of hypoxia. Their activation initiates a number of protective reflexes, including arousal from sleep, sympathetic activation, and increased drive to breathe. The transduction events between hypoxia and increased action potential (AP) activity on the sinus nerve are poorly resolved, but release of an excitatory agent from a cell (glomus cell) presynaptic to the afferent nerve fibers is postulated to be an essential step (20). Ever since the 1920s when the organ function was established, it was known that acetylcholine (ACh) and its derivatives stimulate afferent activity (25). Thus a natural hypothesis was that hypoxia leads to ACh release and, thusly, afferent nerve excitation.
Some experimental data supported this conjecture. For instance, high doses of d-tubocurarine reduced the response to acute hypoxia in cat chemoreceptors, in vitro (6), and hexamethonium, in vivo, reduced the nerve response to asphyxia and cyanide (27). However, results were often inconsistent, even within the same study. For instance, hexamethonium, in vivo, reduced the cat chemoreceptor response to cyanide in 25% of the nerve fibers but had no effect on the response of the other 75% (32). Mecamylamine, at high doses, reduced the response to ACh and cyanide, but at lower doses only reduced the response to ACh and either had no effect on the response to cyanide or increased the response to cyanide (32). Other studies demonstrated a partial reduction in the nerve response to hypoxia by mecamylamine, but these generally used high drug concentrations of mecamylamine (100–640 μM) and whole sinus-nerve recordings, encompassing many unit activities (6, 15, 24). Quantification of multiunit activities using threshold detection (24) (i.e., window discriminator) or nerve root mean square (RMS) voltage (15) is sensitive to AP size as well as AP rate.
The purpose of the present study was to address the potential role of excitatory nicotinic transmission using an experimental configuration in which a high-resolution, single-unit activity may be observed along with changes in the AP morphology and conduction velocity between the carotid body and petrosal ganglion. The results demonstrate that mecamylamine enhances (does not decrease) the AP frequency response to moderate hypoxia, a result incompatible with nicotinic receptors mediating excitatory transmission to the afferent nerve fibers. In addition, mecamylamine reduced AP height and, at high doses, slowed nerve conduction and produced AP conduction failure. Both actions, which are not related to its receptor blocking action, may have contributed to the appearance of reduced chemoreceptor activity in some previous studies.
METHODS
Experiments were undertaken with the approval of the Yale Animal Care and Use Committee.
Animal model.
Experiments were conducted on Sprague-Dawley rat pups of both sexes, between the ages of postnatal day 18 (P18) and P26. The age range was chosen as a period after the time of postnatal maturation of chemoreceptor activity (28) and before the onset of sex hormones. Because of the slow kinetics of drug washout, only a single recording was taken per carotid body.
Experimental preparation for afferent nerve recording.
Before tissue harvest, rats were deeply anesthetized by placement in a closed chamber whose atmosphere was gradually replaced with 100% CO2. While anesthetized, the animals were removed and decapitated. The carotid body/sinus nerve/glossopharyngeal nerve/petrosal ganglion were removed, en block, as previously described (3) and placed in oxygenated (95% O2-5% CO2) saline (in mM: 120 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 1 MgSO4, 24 NaHCO3, and 5 glucose). To aid in tissue cleaning, the complex was transferred to a chamber filled with saline containing collagenase (0.1%, Roche Diagnostics type P) and protease (0.02% Sigma type XIV) at 37°C for 30 min with gentle agitation. The complex was further cleaned and transferred to a perfusion chamber (Warner Instruments RC-22C, chamber volume ∼140 μl) mounted on the stage of an inverted microscope equipped with Hoffman contrast optics (Zeiss Axiovert 10). The complex was superfused with oxygenated (21% O2-5% CO2-balance N2) saline controlled at 37°C by an inline heater (TC344 temperature controller; Warner Instruments). Perfusion rate was ∼3 ml/min and determined by a peristaltic pump (Gilson). The perfusion tubing was stainless steel except for the peristaltic tube and short interconnecting lines. Chamber oxygen tension next to the carotid body was measured by phosphorescence quenching (Oxy-micro with PST-1 probe, World Precision Instruments).
Single-unit activity was recorded using a suction electrode advanced into the petrosal ganglion. Electrode tip size was ∼30 μm in diameter, which allowed individual ganglion cells to enter the tip. The pipette potential was amplified with an extracellular amplifier (BAK Instruments, MDA5), filtered (0.2–5 kHz), displayed on an oscilloscope, digitized (10-kHz sample rate), and stored on computer (Axoscope, Axon Instruments). Unit chemoreceptor activity was discriminated and timed, post hoc, using an event detection program that identified the timing and magnitude of AP events (CLAMPFIT9.0, Axon Instruments). The number of spikes was binned into sequential 5-s periods, graphed against chamber Po2, and fit to a single decreasing exponential function (Origin 7.5; Microcal).
As an aid for identification of nerve fibers projecting to the carotid body and for measurement of conduction velocity, a stimulus electrode (pipette filled with 1 M NaCl; 1-MΩ impedance) was advanced into the carotid body. A constant-current stimulus (200 μA) (BSI-2, BAK Instruments) was delivered at 1 Hz × 0.05 ms (BPG-1, BAK Instruments), and the success of the stimulus in initiating an orthodromic AP was determined in the poststimulus period. Conduction time was measured as the time period from the start of the stimulus artifact to the time of arrival of the afferent spike at the soma.
All drugs were purchased from Sigma. Mecamylamine (50 and 500 μM, final concentration), hexamethonium (100 μM final concentration), methyllycaconitine (10 μM final concentration), and ACh were dissolved in saline. Dosages were based on previous studies that demonstrated an inhibitory effect on the nerve response to hypoxia: mecamylamine, 100 and 500 μM (24); mecamylamine, 490 μM (8); mecamylamine, 67–670 μm (15); and hexamethonium 36–183 μM (6).
Experimental protocol.
The same experimental protocol was followed for low-dose mecamylamine, high-dose mecamylamine, hexamethonium, methyllycaconitine, and suramin. After an initial stabilization period, the response to acute, progressive hypoxia was elicited by switching the perfusate to a small (60 ml) reservoir, initially equilibrated with 21% O2-5% CO2-balance N2 and residing in a heated (37°C) water bath. The reservoir Po2 was decreased by switching the gas source for bubbling to 0% O2-5% CO2-balance N2, resulting in a slow decrease in Po2. The hypoxic stimulus was terminated on reaching 60 Torr or when an obvious decrease in AP rate was observed, indicating a maximal response had already been achieved. For drug administration, the perfusate was switched to a reservoir containing the drug and initially equilibrated with 21% O2-5% CO2-balance N2. A 10-min drug equilibration period was allowed and the response to hypoxia elicited by bubbling the same reservoir with 5% CO2-balance N2. After the hypoxia trial in the presence of drug, the perfusate was switched to a reservoir that was free of drug for 10 min and again bubbled with 5% CO2-balance N2.
Orthodromic APs were elicited for measurement of conduction time and AP height after the initial hypoxia challenge, at ∼9 min into the drug wash-in period and at 9 min into the drug washout period. The response to exogenous ACh was also tested at this time by adding a bolus (50 μl) application of saline containing 10 mM ACh to the inlet of the perfusion chamber.
In other experiments that examined dynamic changes in AP height or conduction time, an electrical stimulus at 2× threshold was delivered at 0.5 Hz. Following a 1-min control period, the perfusate was switched to a reservoir containing the drug for 5 min and then bubbled with 5% CO2-balance N2.
Patch-clamp recording.
Whole cell patch-clamp recordings were obtained for measuring ACh-induced currents during application of ACh directly to the soma of chemoreceptor neurons. The method of initial identification and bringing cells to the surface was previously described (9). Electrodes were fabricated from borosilicate glass and pulled to 1.5–2.2 MΩ when filled with intracellular solution (in mM: 130 KF, 10 HEPES, 5 EGTA, 1 CaCl2, 5 Mg-ATP, pH 7.2). Cells were voltage clamped at −70 mV (HEKA EPC-10 amplifier under control of HEKA Patchmaster program) at 30°C and electrode current measured during 100-ms application (Picospritzer II) of ACh (1 mM) containing saline.
Data analysis.
Unit spiking rates before, during, and following drug applications were recorded from the best-fit exponential line of the AP frequency vs. Po2 graph. Values were taken at normoxia (ca. 150 Torr), 80 Torr, and 70 Torr chamber oxygen tensions. Nerve conduction time was measured as the average time for three electrical stimuli from the stimulus artifact to the arrival of the AP at the soma. AP height was inferred from the extracellular field and measured as the maximum height difference from baseline to the negative-going peak of the AP. Data were initially analyzed using ANOVA with repeated measures and post hoc testing accomplished using paired t-test between control and drug or washout values. Significance was considered at P < 0.05.
RESULTS
Low dosage of mecamylamine (50 μM).
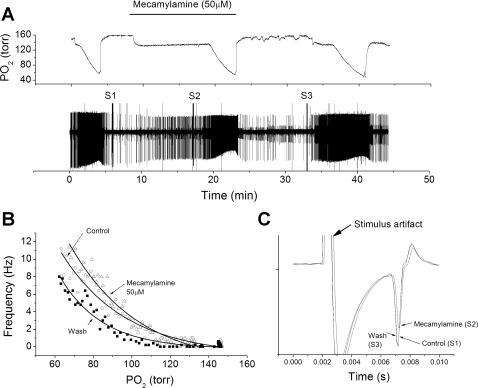
The unit response to progressive hypoxia was elicited by switching the source of gas bubbling through the perfusion reservoir from 21% O2-5% CO2-balance N2 to 0% O2-5% CO2-balance N2 (Fig. 1A). This resulted in a slow decrease in chamber oxygen tension, and the rate of decent could be regulated by the bubbling rate. Unit activity was quantified in 5-s bins, graphed against chamber O2 tension, and best fit to a single-exponential function (Fig. 1B). After the initial response to hypoxia, switching to a reservoir containing 50 μM mecamylamine caused no apparent decrease in spontaneous AP activity. After a 10-min wash-in period, the unit response to hypoxia in the presence of mecamylamine was robust (Fig. 1A), with no apparent decrease in AP rates during progressive hypoxia (Fig. 1B). AP rates during normoxia were not significantly different before, during, and following drug application: 0.47 ± 0.15, 0.98 ± 0.36, and 0.67 ± 0.29 Hz (n = 9), respectively (F = 0.95, df = 2, P = 0.4). AP rate at 80 Torr was significantly affected over the treatment period (F = 6.58, df = 2, P = 0.008) due to a significant increase in AP rate from the control value of 7.4 ± 2.2 to 11.7 ± 2.8 Hz in the presence of low-dose mecamylamine (t = 2.9, P = 0.019). Similarly, the AP rate at 70 Torr was different across the treatment period (F = 5.34, df = 2, P = 0.017) due to a significant increase in AP rate during perfusion with mecamylamine from 9.78 ± 2.7 (control) to 15.16 ± 3.5 (drug) Hz (t = 3.08, P = 0.015).
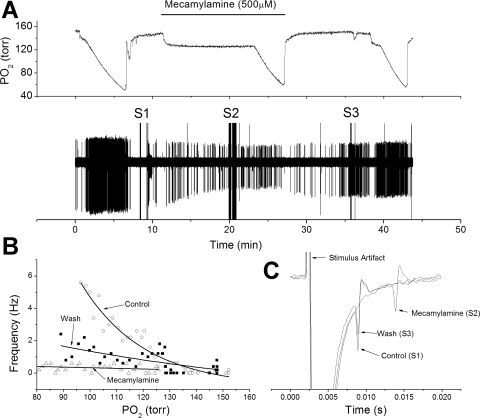
Fig. 1.
Low-dose mecamylamine fails to reduce chemoreceptor nerve response to hypoxia. A: polygraphic recording of experimental run. Top trace: chamber O2 tension. Bar indicates period of mecamylamine perfusion. Bottom trace: raw recording of action potential (AP) activity recorded from petrosal chemoreceptor neuron with time on the abscissa and voltage on the ordinate. S1, S2, and S3 are stimulus artifacts during electrical stimulation to the carotid body for orthodromic AP initiation. B: frequency/Po2 response relationship for chemoreceptor before (○), during (△), and following (■) perfusion with mecamylamine. Exponential line fits using least-squares method. Note increased response to hypoxia in presence of mecamylamine. C: expanded, overlaid traces during orthodromic stimulation from S1 to S3. In this case, mecamylamine slightly reduced AP height but did not change conduction time.
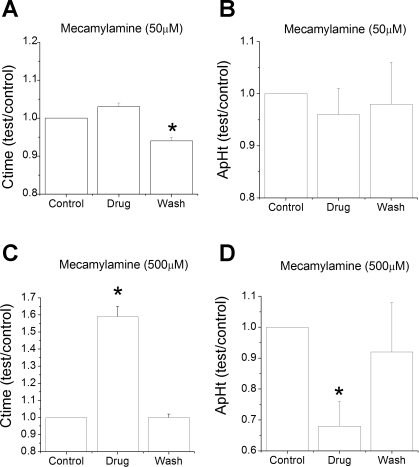
Nerve conduction time significantly changed over the treatment period (F = 16.9, df = 2, P < 0.001). During mecamylamine exposure, conduction time slightly increased from 4.83 ± 0.09 to 4.99 ± 0.15 ms (n = 9) (Fig. 1C), but this change fell slightly below the significance threshold (P = 0.061). After drug washout, conduction time decreased to 4.56 ± 0.13 ms, which was significantly less than the control period (t = 3.8, P = 0.009) or during drug perfusion (see Fig. 4) (t = 5.1, P = 0.002). AP height, as inferred from the extracellular field, did not significantly change over the treatment period (F = 0.49, df = 2, P = 0.6) (see Fig. 4).
Fig. 4.
Normalized conduction time (Ctime) and AP height (ApHt) during and following mecamylamine. Values during drug exposure and following wash are divided by control (predrug) values. A: conduction time trended to be prolonged during exposure to low-dose mecamylamine but fell below significance (P = 0.061). Following washout, conduction time was significantly faster than control. B: low-dose mecamylamine did not significantly change AP height. C: high-dose mecamylamine significantly slowed conduction time, which reversed during washout. D: high-dose mecamylamine significantly reduced AP height, which reversed during washout. Comparisons are paired t-tests vs. control values. *P < 0.05.
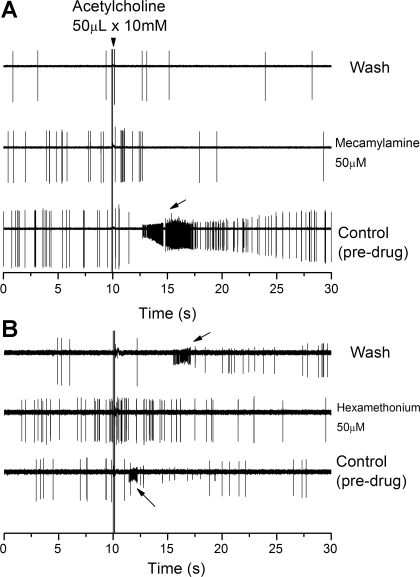
Low-dose mecamylamine effectively blocked the excitatory action of exogenous ACh (see Fig. 8). Before drug application, ACh, directly added to the chamber in a 50-μl bolus (10 mM concentration), caused a burst of AP activity and reduced AP height (see Fig. 8A). The reduced AP height is probably a direct action of ACh on the soma, but the increased AP rate could be an effect at either the nerve terminals or from the somal depolarization. In the presence of mecamylamine, no reduction in AP height or increase in AP rate was observed (see Fig. 8A). Even after the 10-min washout period of the blocker, exogenous ACh failed to alter AP rate or AP height (see Fig. 8A), suggesting a slow dissociation kinetic for mecamylamine from those channels present in chemoreceptor neurons.
Fig. 8.
Mecamylamine and hexamethonium block the excitatory effects of exogenous acetylcholine. A: polygraphic nerve recordings before, during, and following perfusion with mecamylamine (50 μM) with time on the abscissa and voltage on the ordinate. Acetylcholine was applied as a bolus (50 μl × 10 mM) application (arrowhead) and caused a rapid increase in frequency and reduced AP height (arrow). Note that despite a 10-min wash of mecamylamine, the excitatory action of acetylcholine was absent. B: polygraphic nerve recordings before, during, and following perfusion with hexamethonium (50 μM). Acetylcholine was applied as a bolus (50 μl × 10 mM) application and caused a rapid increase in frequency and reduced AP height (arrow). Blocking action of hexamethonium was reversed during washout, suggesting a more rapid dissociation from the receptor compared with mecamylamine.
High dosage of mecamylamine (500 μM).
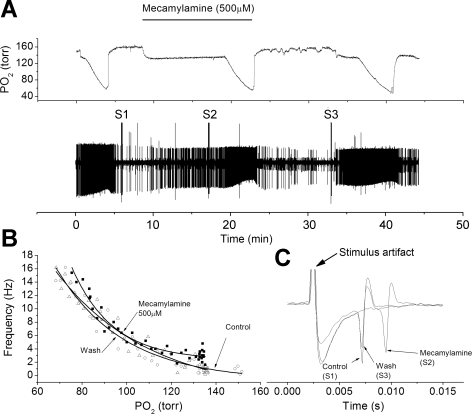
The unit responses in the presence of high doses of mecamylamine were variable. In most units, mecamylamine had no apparent effect on the unit response to hypoxia (Fig. 2, A and B). However, even in these fibers, mecamylamine caused a pronounced slowing of nerve conduction velocity and a reduction in AP height (Fig. 2C). In other units, mecamylamine did not ablate the spontaneous AP activity but did appear to profoundly reduce the AP rate during hypoxia (Fig. 3, A and B). Here, too, mecamylamine also reduced AP height and slowed nerve conduction velocity (Fig. 3C). For the population, high-dose mecamylamine changed conduction time over the treatment period (F = 34.8, df = 2, P < 0.001), and the conduction time during drug exposure was significantly longer than during control (t = 5.9, P = 0.004) (Fig. 4). AP height during drug exposure was also significantly lower than control (t = 5.0, P = 0.004) (Fig. 4). For the population, nerve activities during normoxia were 1.5 ± 0.7 (n = 5), 2.1 ± 0.8, and 1.2 ± 0.9 Hz for control, drug, and washout points and were not different across the treatment period (F = 0.31, df = 2, P = 0.7). AP rates at 80 Torr Po2 were 9.4 ± 1.8, 7.5 ± 1.9, and 10.7 ± 4.3 Hz and were not different across the treatment period (F = 1.2, df = 2, P = 0.3) owing to the high variability of unit responses. AP rates at 70 Torr PO2 were 12.1 ± 2.1, 8.6 ± 2.4, and 12.5 ± 4.7 Hz and were not different across the treatment period (F = 1.6, df = 2, P = 0.25).
Fig. 2.
High-dose mecamylamine fails to reduce chemoreceptor nerve response to hypoxia in some chemoreceptor units. A: polygraphic recording as in Fig. 1. B: frequency/Po2 response relationship before (○), during (△), and following (■) perfusion with mecamylamine (500 μM). Note relatively little effect of mecamylamine on the chemoreceptor response to hypoxia. C: expanded, overlaid traces during orthodromic stimulation. Stimulus intensity was increased between S1 and S2 due to failure to evoke an AP. Mecamylamine slowed conduction velocity and reduced AP height.
Fig. 3.
High-dose mecamylamine appears to reduce chemoreceptor nerve response to hypoxia in some chemoreceptor units. A: polygraphic recordings as in Fig. 1. B: frequency/Po2 response relationship before (○), during (△), and following (■) perfusion with mecamylamine (500 μM). Note greatly reduced AP rate during hypoxia in the presence of mecamylamine. C: expanded, overlaid traces during orthodromic stimulation. Mecamylamine slowed conduction velocity and reduced AP height. It also led to AP propagation failure as observed during S2 (see Fig. 5).
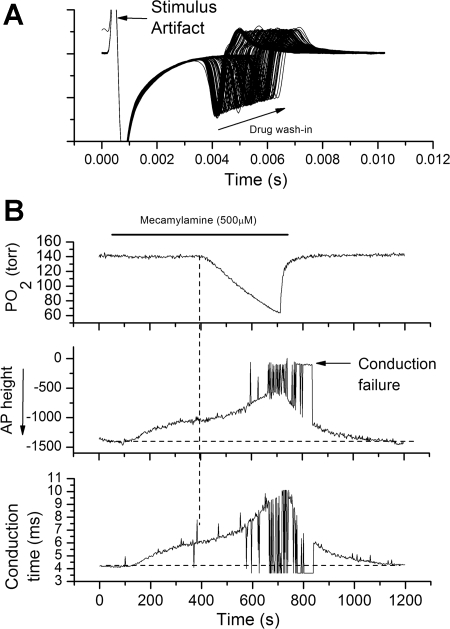
During measurement of conduction time in the presence of mecamylamine, it was often noted that the electrical stimulus failed to evoke an AP. To better examine the action of mecamylamine of AP characteristics, an orthodromic stimulus was repeatedly delivered (0.5 Hz) before, during, and following mecamylamine application in five other units (Fig. 5). During wash-in of mecamylamine (500 μM), AP conduction time increased and AP magnitude decreased (Fig. 5A); application of hypoxia in the presence of mecamylamine caused a further reduction in AP height and further slowed conduction velocity (Fig. 5B). In addition, in a large percentage of the trials, the electrical stimulus failed to elicit an orthodromic AP, despite having the stimulus intensity set at 2× original threshold (Fig. 5B). This suggests AP conduction failure. Switching back to normoxia-equilibrated saline reversed the conduction and AP height changes (Fig. 5B).
Fig. 5.
High dose of mecamylamine reduces AP height, slows AP velocity, and leads to AP propagation failure during hypoxia. A: overlaid polygraphic sweeps during application of stimulus pulses to the carotid body during 5-min wash-in of mecamylamine (arrow). Time is on the abscissa and voltage on the ordinate. Mecamylamine slowed nerve conduction and reduced AP height. B: hypoxia (vertical dashed line) in the presence of mecamylamine further slows conduction velocity, reduces AP height, and leads to AP conduction failure. AP height and conduction time were determined by peak detection program (Clampfit9) in the poststimulus period. Conduction failure is indicated by a near zero AP height. AP detection and height were measured in the negative-going phase in the 10-ms poststimulus period.
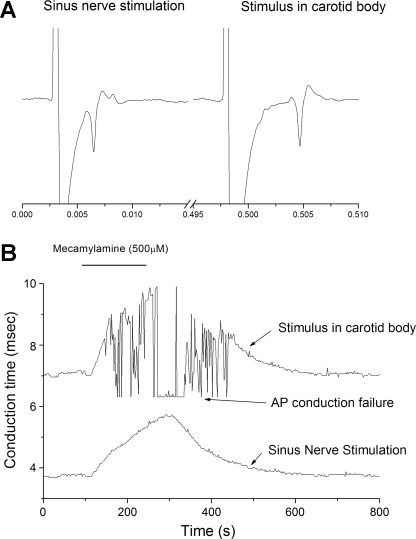
To better identify the site of AP conduction failure, a second stimulus electrode was positioned on the sinus nerve at its entry point to the carotid body and alternating stimuli delivered to the two electrodes (n = 3). As expected, conduction time was less for AP initiated by direct sinus nerve stimulation (Fig. 6). Addition of mecamylamine (500 μM) lengthened conduction time from both stimuli, but conduction failure was only noted for AP arising from stimuli applied within the carotid body, suggesting that the site of AP conduction failure was within the carotid body and not elsewhere in the conduction pathway (Fig. 6).
Fig. 6.
AP conduction failure occurs within the carotid body. One stimulus electrode was placed within the carotid body and one directly on the sinus nerve. Each electrode was stimulated at 1/s with 0.5-s delay between electrodes. A: polygraphic tracing following stimuli directly delivered to the sinus nerve (left) or carotid body (right). B: conduction time between the stimulus and arrival of the AP at the soma. Addition of mecamylamine prolonged nerve conduction time at both sites, but AP conduction failure only occurred for stimulus delivered in the carotid body.
Effect of hexamethonium on AP activity.
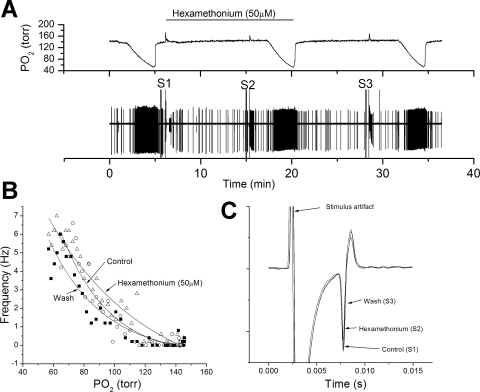
Hexamethonium (50 μM) was tested in four preparations, using the same protocol as that used for mecamylamine. Hexamethonium had no discernable action on the AP rate response to hypoxia (Fig. 7B), conduction time (Fig. 7C), or AP amplitude (Fig. 7C). The drug antagonized the excitatory action of exogenous ACh but washed out more readily than mecamylamine (Fig. 8B). After the 10-min washout period, exogenous ACh again reduced AP height, a recovery not observed with mecamylamine.
Fig. 7.
Hexamethonium fails to reduce chemoreceptor nerve response to hypoxia. A: polygraphic recording as in Fig. 1. B: frequency/Po2 response relationship before (○), during (△), and following (■) perfusion with hexamethonium (50 μM). Note relatively little effect of hexamethonium the chemoreceptor response to hypoxia. C: expanded, overlaid traces during orthodromic stimulation. Hexamethonium had no effect on AP height or conduction velocity.
ACh-induced current in chemoreceptor neurons.
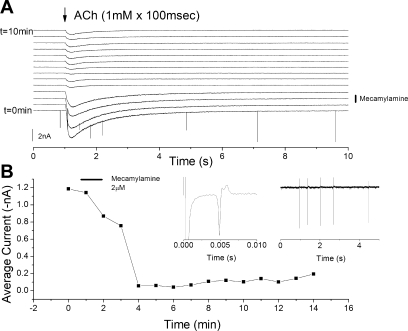
To better examine the inhibitory action and washout of mecamylamine, whole cell recordings were undertaken at the soma of identified chemoreceptor neurons (n = 5) (Fig. 8). Spritzing (100-ms duration, 1/min) of saline containing ACh (1 mM) onto the soma induced an inward current. Addition of mecamylamine (2 μM) to the chamber superfusate for 1 min inhibited the inward current associated with ACh application, and recovery of the ACh-induced current was only partial over a 10-min washout period (Fig. 9). Thus, even with millimolar ACh concentration, a low dose of mecamylamine reduced the ACh current by >90% for a period of minutes.
Fig. 9.
Blocking action of mecamylamine slowly reverses during washout. A: current records of patch-clamp recording from soma of petrosal chemoreceptor neuron during spritzing (1 mM × 100-ms duration) of acetylcholine to the soma. Mecamylamine was applied at 2 μM concentration for 1 min, as indicated. B: average acetylcholine-induced current as calculated between 1 s (time of application) and 4 s. Note rapid decrease in acetylcholine-induced current and slow recovery. B, left inset: orthodromic AP in recorded neuron following an electrical stimulus to the carotid body. B, right inset: spontaneous irregular AP activity recorded from same neuron.
Effect of methyllycaconitine on chemoreceptor response to hypoxia.
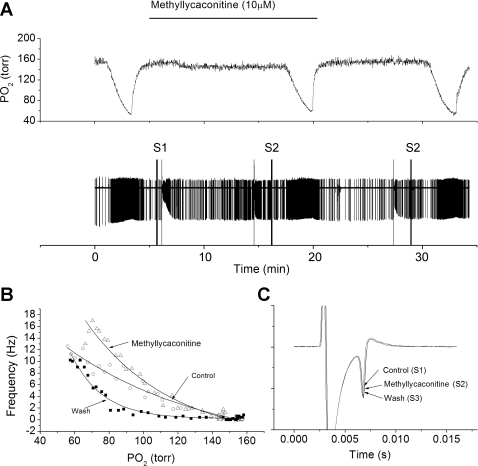
The primary α-subunit isoform in cat carotid body is α7 (38), and the mRNA for the α7 isoform is expressed in rat carotid body and petrosal ganglia (17). The effect of the specific nicotinic receptor α7 antagonist, methyllycaconatine, was tested in three preparations. In all instances, methyllycaconatine failed to reduce the nerve activity during hypoxia compared with the control response (Fig. 10B), and was also without effect on nerve conduction time or AP amplitude (Fig. 10C).
Fig. 10.
Methyllycaconitine, a blocker of the α7 nicotinic receptor, fails to reduce chemoreceptor nerve response to hypoxia. A: polygraphic recording as in Fig. 1. B: frequency/Po2 response relationship before (○), during (△), and following (■) perfusion with methyllycaconitine (10 μM). Note hypoxic responsiveness was maintained in the presence of blocker. C: expanded, overlaid traces during orthodromic stimulation. Methyllycaconitine had no effect on AP height or conduction velocity.
Effect of suramin on chemoreceptor response to hypoxia.
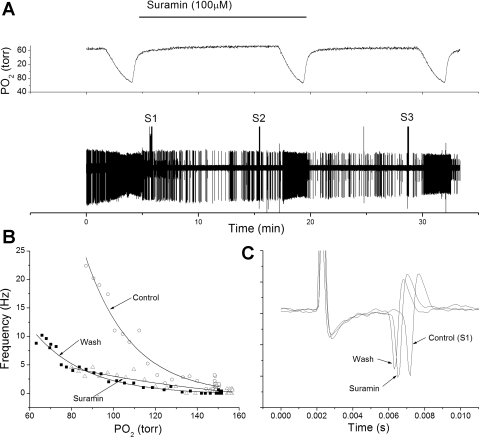
One concern was the ability of drugs, such as mecamylamine, to permeate the carotid body tissue and reach the presumed synaptic site between glomus cell and nerve ending. While direct assessment is difficult, the action of the P2X receptor blocker, suramin, was tested in four preparations. Suramin has been previously demonstrated to reduce spontaneous AP chemoreceptor activity during stop flow (39) and has a larger molecular weight (MW 1,429) than mecamylamine (MW 203). Diffusion through a solute is a smooth increasing function of molar volume, suggesting that diffusion of suramin should be slower than mecamylamine. In all cases, suramin reduced, but did not abolish, the response to hypoxia without changing AP height (Fig. 11). This suggests that drugs used in this preparation had relatively free access to the AP generation site within the carotid body.
Fig. 11.
Suramin, a blocker of P2X receptors, reduces the chemoreceptor response to hypoxia. A: polygraphic recording, as in Fig. 1. B: frequency/Po2 response relationship before (○), during (△), and following (■) perfusion with suramin (100 μM). Note greatly reduced AP rate during hypoxia in the presence of suramin. C: expanded, overlaid traces during orthodromic stimulation. Suramin had little effect on AP height and did not slow conduction time. The inhibitory action of suramin suggests free diffusion of the drug to glomus cells with the carotid body.
DISCUSSION
The primary conclusion from the present work is that the lower doses of ganglionic blocking agents have no discernable inhibitory action on rat chemoreceptor responses to acute hypoxia. This result is inconsistent with nicotinic receptors critically mediating excitatory transmission from glomus cell to nerve ending in rat carotid body. Moreover, higher doses of mecamylamine slowed nerve conduction velocity, reduced AP height, and led to AP conduction failure. Thus mecamylamine, and perhaps other ganglionic blocking agents, may produce apparent changes in chemoreceptor activity that are not related to its ganglionic blocking action and that complicate interpretation of previous studies that relied on whole nerve recording techniques.
Ganglionic blocking agents and chemoreceptor function.
The stimulatory action of ACh and its derivatives on peripheral chemoreceptors has been known almost as long as the organ function was established (26). This naturally gave rise to the hypothesis that ACh release was causal to the increase in nerve activity. If so, then one corollary is ganglionic blocking drugs should reduce or ablate the chemoreceptor response to hypoxia. This conjecture was extensively examined using in vivo models in which chemoreceptor activity was either directly measured or indirectly measured through ventilatory changes. Most results failed to support an excitatory role for nicotinic receptors in the response to hypoxia or cyanide. For instance, hexamethonium (4) and mecamylamine (30, 37), in vivo, blocked the cat chemoreceptor response to exogenous ACh but had no effect on the response to anoxia or cyanide. Similar results were found in dogs treated with tetraethylammonium as an ACh antagonist (31). Other results showed partial or variable effects among chemoreceptor units. Nishi and Eyzaguirre (32) observed that hexamethonium inhibited the response of cat chemoreceptors to cyanide, but this only occurred in 25% of the chemoreceptors and had no effect on 75%. Similarly, Paintal (24) observed that exogenous ACh stimulated unmyelinated aortic chemoreceptor fibers but had little effect on myelinated chemoreceptors, leading that author to conclude that ACh was not an essential transmitter since both fibers responded similarly to hypoxia.
Other data demonstrate that ganglionic blocking agents impair the chemoreceptor response hypoxia. Generally, these studies utilized whole nerve recordings and high doses of blocking agents. For instance, in rat carotid body, He et al. (24) demonstrated that mecamylamine (100 μM) reduced the whole nerve response to acute hypoxia (21% O2) by 80%. In cat carotid body and using an in situ perfusion system, Fitzgerald et al. (15) demonstrated that mecamylamine (67–640 μM) reduced the response to hypoxia in a dose-dependent manner, resulting in about half-inhibition at 640 μM. Similarly, Eyzaguirre and colleagues demonstrated that high doses of d-tubocurarine (6) and mecamylamine (ca. 500 μM) (7) reduced the nerve response to hypoxia, in vitro. Since the functional Kd for mecamylamine is ∼340 nM (19), the concentrations used for these studies were 200–2,000 times the Kd for the receptor. However, high dosage may be justified by the argument that synaptic concentration of ACh could reach millimolar levels and, thus, require high doses of blocker to antagonize the effect (11). It is from this perspective that we consider several aspects of AP generation by cholinergic transmission.
Excitatory postsynaptic potential model.
In this schema, transmission between glomus cells and nerve endings by ACh or other excitatory agents is by episodic events, i.e., synaptic depolarizing potentials that may summate and give rise to APs. This schema is consistent with data from sharp-electrode recordings of chemoreceptor nerve terminals (22) and data demonstrating depolarization events in a coculture model of glomus cells and petrosal neurons (42). Thus ACh could be acting singly or in conjunction with other excitatory transmitters to produce depolarization events.
In this model, one would not expect any difficulty for ganglionic blocking agents to antagonize the effects of ACh. For most of the time, particularly when AP rates are low, there would be no competition for binding between ACh and the ganglionic blocking drugs. Thus, while high doses of blocking agent may be required if competing with high levels of ACh, this would only occur episodically. Instead, the endogenously released ACh would need to wait for the blocking agent to leave the receptor, which, for mecamylamine, may be 10–30 min. In this present study, no recovery of blocking action following 50 μM mecamylamine was observed after a 10-min washout period. And following a short application (1 min) of 2 μM mecamylamine, ACh-induced inward currents in chemoreceptor neurons only slightly recovered after a 10-min washout period. A similar slow recovery is observed in rat chromaffin cells in which recovery from 1 μM mecamylamine is only 32% complete after 10-min washout (16). In an expression system, the time constant of recovery from mecamylamine was 34 min for the α4β2, 32 min for the α3β2, and 19 min for the α4β4 isoforms (35).
An excitatory postsynaptic potential-like transmission based on ACh is also not supported by anatomic evidence or based on ACh release. ACh tissue efflux from cat carotid body, as measured with HPLC, increased from 0.6 pmol/150 μl in normoxia (21% O2-6% CO2) to 0.8 pmol/150 μl with hypoxia (0% O2-5% CO2), a ratio of 1.3 (16). A similar augmentation of ACh release was recently observed in rat carotid bodies (14). The anticipated increase in nerve activity based on nerve recordings would be from roughly 1 Hz to 20 Hz (12, 13). If ACh release evokes a synaptic depolarizing potential (SDP) that initiates an AP with a safety factor, as occurs at the neuromuscular junction, then changes in nerve activity should be directly proportional to ACh release, but this is clearly not the case. If AP generation requires the summation of multiple SDPs, such a process would have a high sensitivity to blocking agents (well below the Kd) whose action would be to change the number of SDPs required to reach threshold. The probability of more simultaneous events decreases exponentially, as specified by the Poisson equation for calculating the probability of simultaneous random events. The problem is even less tractable in the rabbit in which the release of ACh decreases by 68% during hypoxia (29), leading to an unlikely speculation that in nature excitatory transmitters are switched among species.
Anatomically, there is also little evidence for the necessary anatomic substrate for SDP producing synapses. Fusion profiles on glomus cells, fixed in high-K+ solution to stimulate secretion, occur over the entire glomus cell surface, bearing no particular relationship to the nerve endings (21). While glomus cell/nerve ending synapses have been described, they appear to lack any consistent clustering of synaptic vesicles, and pre/post-synaptic morphology varies from species, leading Verna to write, “I wonder if it is possible to say that the existence of a chemical synapse between glomus cells and sensory nerve endings is really demonstrated!” (40). This anatomic variability would be surprising in a synaptic site requiring high local concentrations of released transmitters for functionality.
Neuromodulator model.
ACh may be acting more as a modulator of excitability and not as a producer of depolarization events. Previously, we (2) and others (5) have reported evidence against the occurrence of SDP events in rat chemoreceptor nerve terminals. Instead, we propose a spike generation process endogenous to the nerve terminals and supported by a persistent sodium current (2, 9, 10). If true, then ACh may be more of a modulator of activity rather than an initiator of APs. This role seems more consistent with the staining profiles for the α7 subunits of the nicotinic receptor that stain long stretches of the chemoreceptor nerve fibers with little clustering, as would be expected at a specialized synaptic site (38). Although these receptors undoubtedly exist and lead to AP stimulation during application of exogenous ACh, it is unclear what role they play under normal physiological conditions. Ganglionic blockers should have access to these sites and would, because of the wide distribution of nicotinic receptor, not be competing with high doses of agonist. Since mecamylamine and hexamethonium at low doses had little effect on AP spiking rate in hypoxia, the modulatory role under normal conditions seems slight.
Potential difficulties of drug permeation.
Critical to acceptance of the conclusion that nicotinic receptors do not mediate excitatory transmission in the carotid body is the ability of the drug to get to the synaptic site. This has been offered as a possible explanation as to why previous investigators were able to block the effects of exogenous ACh, which may stimulate extrasynaptic sites, but not block the excitatory actions of cyanide or hypoxia (15). While the permeation of mecamylamine was not directly assessed, suramin, a P2X receptor blocker, was used as an agent with larger molecular weight. P2X receptors have been implicated as serving a critical role in chemoreceptor AP generation (36, 39, 41). Here, application of suramin in the same time frame was effective in greatly reducing the excitatory effect of hypoxia, suggesting little difficulty for the drug to reach the site of AP generation. This is consistent with the previous observations that horseradish peroxidase, a large-molecular-weight substance, applied through the blood vessels, rapidly appeared in the area around the nerve endings/glomus cell interface (1).
Nonspecific actions of ganglion blocking agents.
In the present study, mecamylamine significantly prolonged nerve conduction time and reduced the height of the AP as measured by the extracellular field. The latter effect would reduce the apparent rate of AP generation when such rate is quantified by the RMS voltage field present on the nerve or by using a threshold discriminator, in which case, fewer APs would surpass threshold. The problem is compounded during hypoxia, which also reduces AP height, probably through a use-dependent block as spontaneous AP rate increases during hypoxia (Figs. 1A and 2A). The combined effects of hypoxia and mecamylamine yield AP propagation failure, which contribute to reduced AP spiking rates observed during mecamylamine plus hypoxia. Nevertheless, it is difficult to quantify the degree of AP propagation failure, which varies among units, and, thus, it is impossible to conclude that the lower response to hypoxia observed in the presence of mecamylamine (Fig. 3B) is solely due to propagation failure. This, however, is the simplest explanation since 1) a reduction in AP activity was not observed during hypoxia at a lower concentration of mecamylamine (50 μM), which is still well above the Kd; and 2) some units showed no reduction in spiking activity at the high dose of mecamylamine (Fig. 2). Unless the chemical nature of the excitatory coupling varies from unit to unit within the same carotid body, the pharmacological effects would be expected to be relatively uniform.
A reconciliation with all previous observations is clearly beyond the present data set, and changes in AP morphology caused by mecamylamine do not explain the actions of other ganglionic blockers. For instance, Varas et al. (39), using intracellular recordings from petrosal neurons, observed a 50% reduction in AP generation during “stop flow” while perfusing with 10 μM hexamethonium. In the present study, hexamethonium had no effect on AP morphology, conduction time or the response to hypoxia. Previous work using whole sinus nerve recordings from rats, demonstrated that methyllycaconatine used at a lower concentration than the present study inhibited the nerve response to hypoxia by 50% (23). Since methyllycaconatine at higher concentration had no effect on AP morphology or conduction time, changes in AP characteristics cannot account for the decrease in activity. A reconciliation may, thus, lie in the specific experimental circumstances. For instance, “stop flow” is not well defined in terms of the hypoxic level achieved or the contribution of metabolic factors that are normally washed out. In the study of He et al. (23), “hypoxia” was achieved by reducing chamber Po2 from 450 Torr to 120 Torr, which is largely above the Po2 levels considered in the present study, and they also used a reduced extracellular chloride concentration with 42 mM chloride replaced by glutamate. A determination of the importance of these differences requires further experiments.
There are numerous other aspects to determining the role of ACh within the carotid body, including the presence and role of autoreceptors on the glomus cells, role of muscarinic receptors, and effect of drugs that alter synthesis and release of ACh. At present, it is even uncertain whether ACh is synthesized in rat glomus cells with evidence for (33) and against (18) synthesis. The issues have been extensively reviewed by Fitzgerald (11). Since the present results shed no light on these other interventions they are not considered here, but need to be considered when evaluating the cholinergic hypothesis, as a whole.
Conclusion.
The present results demonstrate that ganglionic blocking agents, mecamylamine and hexamethonium, at lower concentrations, have no significant effect on the generation rate of carotid body AP activity. Thus ACh, acting through nicotinic receptors, is unlikely to be the major or a major excitatory transmitter between glomus cells and nerve endings in the carotid body. At high doses, mecamylamine reduces AP height and leads to AP conduction failure, particularly during hypoxia, which may contribute to the apparent change in chemoreceptor activity observed under some experimental conditions. Thus extra caution is warranted in interpreting experimental data that may be sensitive to these extraganglionic effects.
GRANTS
This work supported by National Heart, Lung, and Blood Institute Grant HL-073500.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1. Bock P. Distribution of intravenously injected horseradish peroxidase in the carotid body of the mouse. Arzneimittelforschung 23: 1613, 1973. [Google Scholar]
- 2. Donnelly DF. Orthodromic spike generation from electrical stimuli in the rat carotid body—implications for the afferent spike generation process. J Physiol 580: 275–284, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J Appl Physiol 88: 1489–1495, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Douglas W. The effect of a ganglion-blocking drug, hexamethonium, on the response of the cat's carotid body to various stimuli. J Physiol 118: 373–383, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eyzaguirre C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol Res 38: 341–345, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Eyzaguirre C, Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J Physiol 178: 410–437, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eyzaguirre C, Zapata P. A discussion of possible transmitter or generator substances in carotid body chemoreceptors. In: Arterial Chemoreceptors, edited by Torrance R. Oxford: Alden and Mowbray, 1968, p. 213–247 [Google Scholar]
- 8. Eyzaguirre C, Zapata P. Pharmacology of pH effects on carotid body chemoreceptors in vitro. J Physiol 195: 557–588, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faustino EV, Donnelly DF. An important functional role of persistent Na+ current in carotid body hypoxia transduction. J Appl Physiol 101: 1076–1084, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Faustino EV, Donnelly DF. Lamotrigine and phenytoin, but not amiodarone, impair peripheral chemoreceptor responses to hypoxia. J Appl Physiol 101: 1633–1640, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Fitzgerald RS. Oxygen and carotid body chemotransduction: the cholinergic hypothesis—a brief history and new evaluation. Respir Physiol 120: 89–104, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald RS, Dehghani GA. Neural responses of the cat carotid and aortic bodies to hypercapnia and hypoxia. J Appl Physiol 52: 596–601, 1982. [DOI] [PubMed] [Google Scholar]
- 13. Fitzgerald RS, Parks DC. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respir Physiol 12: 218–229, 1971. [DOI] [PubMed] [Google Scholar]
- 14. Fitzgerald RS, Shirahata M, Chang I, Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res 1270: 39–44, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald RS, Shirahata M, Ide T. Further cholinergic aspects of carotid body chemotransduction of hypoxia in cats. J Appl Physiol 82: 819–827, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald RS, Shirahata M, Wang HY. Acetylcholine release from cat carotid bodies. Brain Res 841: 53–61, 1999. [DOI] [PubMed] [Google Scholar]
- 17. Gauda EB. Gene expression in peripheral arterial chemoreceptors. Microsc Res Tech 59: 153–167, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Gauda EB, Cooper R, Johnson SM, McLemore GL, Marshall C. Autonomic microganglion cells: a source of acetylcholine in the rat carotid body. J Appl Physiol 96: 384–391, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Giniatullin RA, Sokolova EM, Di Angelantonio S, Skorinkin A, Talantova MV, Nistri A. Rapid relief of block by mecamylamine of neuronal nicotinic acetylcholine receptors of rat chromaffin cells in vitro: an electrophysiological and modeling study. Mol Pharmacol 58: 778–787, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. [DOI] [PubMed] [Google Scholar]
- 21. Gronblad M. Improved demonstration of exocytotic profiles in glomus cells of rat carotid body after perfusion with glutaraldehyde fixative containing a high concentration of potassium. Cell Tissue Res 229: 627–637, 1983. [DOI] [PubMed] [Google Scholar]
- 22. Hayashida Y, Koyano H, Eyzaguirre C. An intracellular study of chemosensory fibers and endings. J Neurophysiol 44: 1077–1088, 1980. [DOI] [PubMed] [Google Scholar]
- 23. He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol 100: 157–162, 2006. [DOI] [PubMed] [Google Scholar]
- 24. He L, Dinger B, Fidone S. Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J Appl Physiol 98: 614–619, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Heymans C, Bouckaert JJ, Dautrebande L. Sinus carotidien et reflexes respiratoires: sensibilite des sinus carotidiens aux substances chimiques. Action stimulante respiratoire reflexe du sulfure de sodium, du cyanure de potassium, de la nicotine et de la lobeline. Arch Int Pharmacodyn Ther 40: 54–91, 1931. [Google Scholar]
- 26. Heymans J, Heymans C. Sur les modifications directes et sur la regulation reflexe de L'activite du centre respiratoire de la tete isolee du chien. Arch Int Pharmacol 33: 273–370, 1927. [Google Scholar]
- 27. Joels N, Neil E. Carotid glomus chemosensory excitation. J Physiol 164: 11P–12P, 1962. [Google Scholar]
- 28. Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol 453: 461–473, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim DK, Prabhakar NR, Kumar GK. Acetylcholine release from the carotid body by hypoxia: evidence for the involvement of autoinhibitory receptors. J Appl Physiol 96: 376–383, 2004. [DOI] [PubMed] [Google Scholar]
- 30. McQueen DS. A quantitative study of the effects of cholinergic drugs on carotid chemoreceptors in the cat. J Physiol 273: 515–532, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moe GK, Capo LR, Peralta B. Action of tetraethylammonium on chemoreceptor and stretch receptor mechanisms. Am J Physiol 153: 601–605, 1948. [DOI] [PubMed] [Google Scholar]
- 32. Nishi K, Eyzaguirre C. The action of some cholinergic blockers on carotid body chemoreceptors in vivo. Brain Res 33: 37–56, 1971. [DOI] [PubMed] [Google Scholar]
- 33. Nurse CA, Zhang M. Acetylcholine contributes to hypoxic chemotransmission in co-cultures of rat type 1 cells and petrosal neurons. Respir Physiol 115: 189–199, 1999. [DOI] [PubMed] [Google Scholar]
- 34. Paintal AS. Mechanism of stimulation of aortic chemoreceptors by natural stimuli and chemical substances. J Physiol 189: 63–84, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther 297: 646–656, 2001. [PubMed] [Google Scholar]
- 36. Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23: 11315–11321, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sampson SR. Effects of mecamylamine on responses of carotid body chemoreceptors in vivo to physiological and pharmacological stimuli. J Physiol 212: 655–666, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shirahata M, Ishizawa Y, Rudisill M, Schofield B, Fitzgerald RS. Presence of nicotinic acetylcholine receptors in cat carotid body afferent system. Brain Res 814: 213–217, 1998. [DOI] [PubMed] [Google Scholar]
- 39. Varas R, Alcayaga J, Iturriaga R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res 988: 154–163, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Verna A. The mammalian carotid body: morphological data. In: The Carotid Body Chemoreceptors, edited by Gonzalez C. Heidelberg, Germany: Springer, 1997, p. 1–29 [Google Scholar]
- 41. Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525: 143–158, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol 503: 599–612, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]