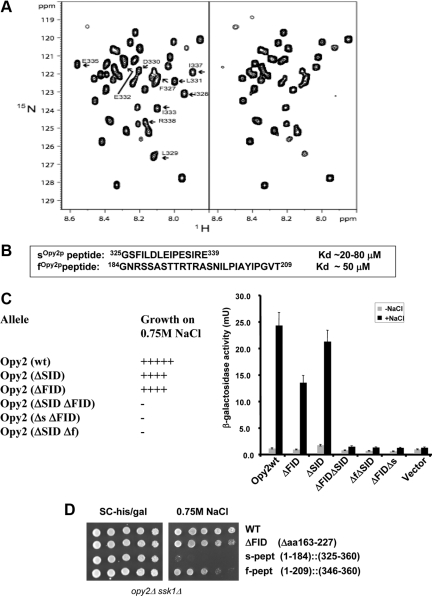
Figure 4.
Two peptide motifs of Opy2p interact with the Ste50p-RA domain. (A) The HSQC spectra of 15N-labeled Opy2p SID (aa 267-345) in the absence (left) or presence (right) of the Ste50p-RA domain. Arrows indicate Opy2p residues involved in Ste50p-RA domain binding. (B) The affinity of synthesized s- and f-peptides for the Ste50p-RA domain. (C) Ability of yeast cells (opy2Δ ssk1Δ) transformed with OPY2 plasmids with indicated deletions to grow (+) or not (−) on hyperosmolarity media (left) and the capacity to activate a transcriptional reporter (8xCRE-CYC1-LacZ) for the HOG pathway (right). Δf (=Δaa185-209), Δs (=Δaa325-339). (D) The f-peptide but not the s-peptide is sufficient to complement the function of the Opy2p cytoplasmic tail. Yeast cells of opy2Δ ssk1Δ transformed with the OPY2 plasmids indicated were assayed for their ability to grow on hyperosmolarity media.