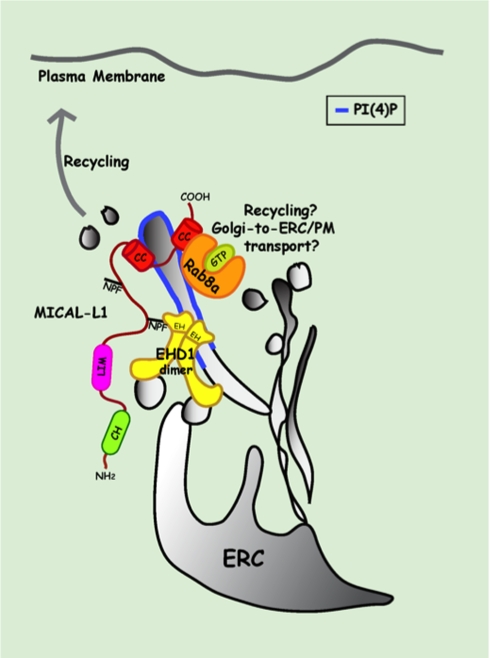
Figure 8.
Potential model depicting MICAL-L1 interactions with EHD1 and Rab8a on tubular recycling endosome membranes. MICAL-L1 is recruited to the membranes of tubular recycling endosomes (blue bars) by its C-terminal CC region. Through the binding of its first NPF motif with the EHD1 EH domain (EH1), it recruits and/or stabilizes EHD1 on these tubules. EHD1 is further stabilized on these membranes by the ability of EH1 to directly bind phosphoinositides, specifically PI4P. MICAL-L1 also directly binds to GTP-bound Rab8a and recruits/and or stabilizes it on the tubular membranes; such binding might be simultaneous or independent of EHD1. Localization of EHD1 to the tubular recycling compartment facilitates receptor recycling from the ERC back to the plasma membrane. Rab8a localization to tubular membranes may also play a minor role in EHD1 and MICAL-L1–mediated endocytic recycling pathway. However, as both EHD1 and Rab8a have been implicated in trafficking from the Golgi compartment, their association through MICAL-L1 might be important for trafficking of cargo from Golgi to the plasma membrane or Golgi-to-ERC transport. ERC, endocytic recycling compartment; PI4P, phosphotidylinositol-4-phosphate.