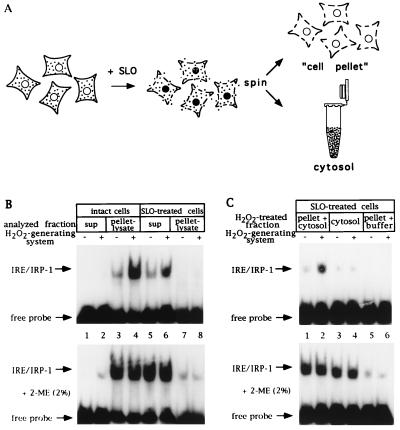
Figure 1.
A cell-free assay for IRP-1 activation by H2O2 based on SLO-permeabilized cells; requirement for nonsoluble factor(s). (A) Schematic representation of the SLO-permeabilization procedure. (B) Fibroblasts (107 control and 107 SLO-permeabilized B6) were suspended in 250 μl SLO buffer, tumbled for 20 min at 37°C, and treated with an H2O2-generating system (see Materials and Methods) for 1 hr. Following centrifugation, 10 μl of supernatants (2.5 μg/μl) and pellet lysates (25 μg protein) were analyzed by EMSA with 25,000 cpm radiolabeled IRE probe without (Upper) or with (Lower) 2% 2-ME. Lanes: 1, 2, 5, and 6, supernatants; 3, 4, 7, and 8, pellet lysates (in cytoplasmic lysis buffer) of intact and SLO-permeabilized cells treated without or with H2O2. (C) Fibroblasts (107 B6) were permeabilized with SLO as in B. Cytosol was separated by gentle centrifugation (supernatant) and cell pellets were washed twice with SLO buffer. Cytosol alone and cell pellets, mixed with one cytosol equivalent or resuspended in buffer, were treated, or not, with H2O2 for 1 hr. Following centrifugation, 10 μl of supernatants (2.5 μg/μl) were analyzed by EMSA with 25,000 cpm radiolabeled IRE probe without (Upper) or with (Lower) 2% 2-ME. Lanes: 1 and 2, cell pellet mixed with cytosol; 3 and 4, cytosol alone; 5 and 6, cell pellets resuspended in buffer, treated without or with H2O2. Positions of IRE/IRP-1 complexes and excess free probe are indicated by arrows.