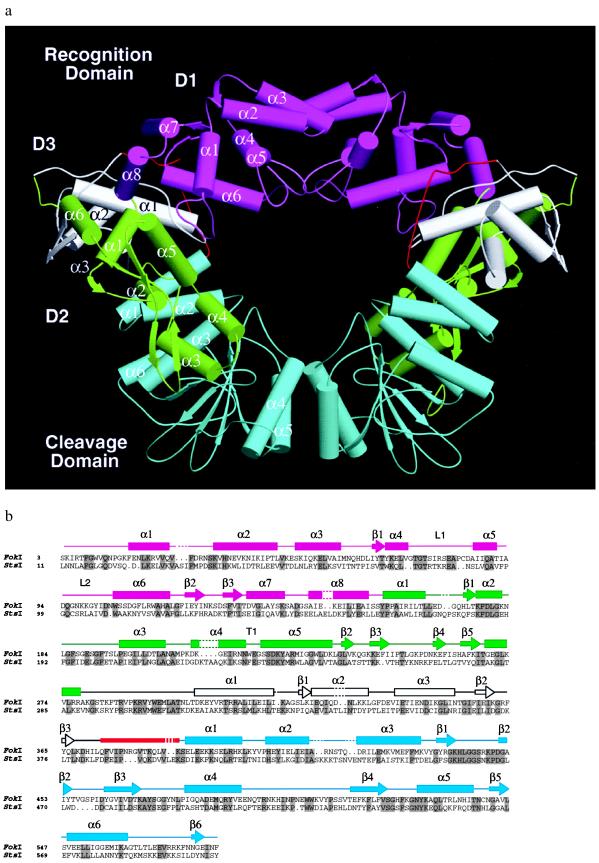
Figure 2.
(a) Structure of the FokI dimer. The recognition domain consists of three smaller subdomains, D1, D2, and D3 shown in magenta, green, and white, respectively. The cleavage domain (blue) is connected to the recognition domain through a linker segment (red). Dimerization is mediated by the parallel helices α4 and α5 of the cleavage domain. There are no significant interactions between the D1 subdomains despite the appearance of such in this orientation of the structure. (b) Secondary structure assignment of FokI. The FokI amino acid sequence is aligned to that of StsI, another type IIs restriction enzyme. StsI recognizes the same sequence as FokI (Fig. 1) but cleaves 10 and 14 bp away. FokI and StsI show 30% sequence identity (residues in gray columns). Dashes in the secondary structure and periods in the sequence represent gaps introduced for optimal alignment.