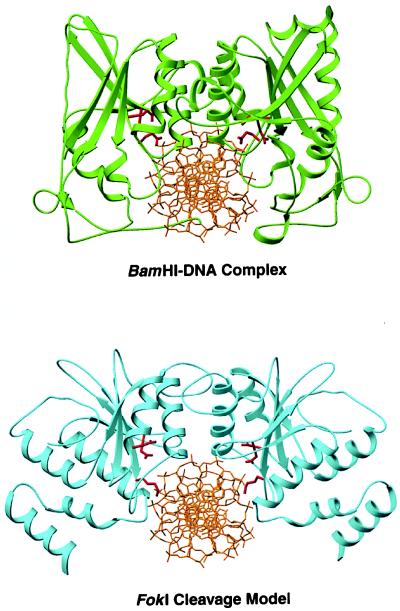
Figure 5.
Comparison of the BamHI–DNA complex (18) with a model of the cleavage domain dimer bound to the FokI cleavage site. Active site residues Asp-94, Glu-111, and Glu-113 in BamHI and Asp- 450, Asp-467, and Lys-469 in FokI are in red. In both structures, the dimerization helices point into the major groove and are roughly perpendicular to it. The FokI dimer provides a prominent cleft for the DNA with the two active sites in close proximity to the scissile bonds.