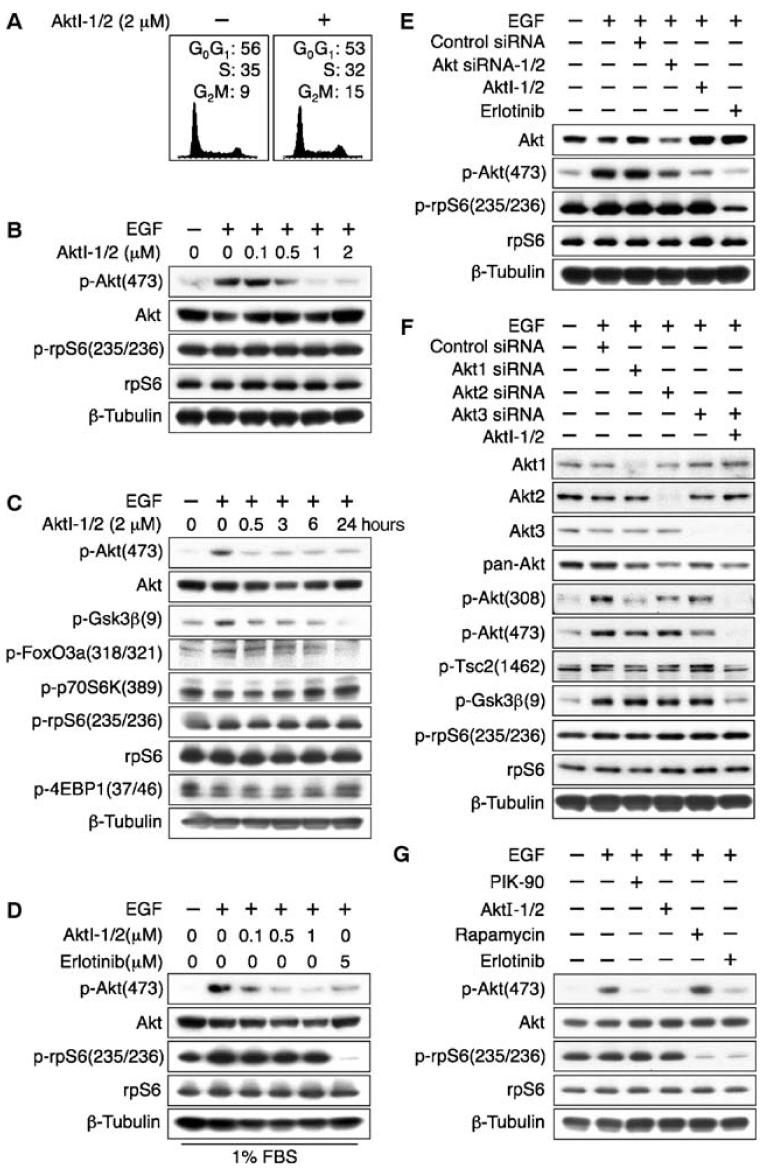
Fig. 2.
Small-molecule inhibition or siRNA knockdown of Akt isozymes fails to affect phosphorylation of the mTOR target rpS6 in PTENwt LN229:EGFR cells. LN229:EGFR cells were treated for 24 hours with indicated dosages of the EGFR inhibitor erlotinib, AktI-1/2 [a PH-domain- dependent isozyme selective inhibitor of Akt1/2 (9)], or siRNA directed against Akt1, Akt2, and Akt3. EGF (50 ng/ml) was added 15 min before harvest, and lysates were immunoblotted. (A) AktI-1/2 (2 μM) had no effect on cell cycle distribution.(B and C)AktI-1/2(2 μM) decreased phosphorylation of Akt and that of substrates of Akt, but had no effect on the abundance of p-p70S6K and p-rpS6. (D) Addition of EGF to cells grown in 1% FBS led to increased p-rpS6 abundance. Inhibition of Akt1/2 had little effect on the abundance of p-rpS6, whereas inhibition of EGFR markedly decreased phosphorylation of this mTOR target protein. (E) The effects of siRNA directed against Akt1/2 were consistent with results obtained with inhibitors of AktI1/2, showing knockdown of Akt and p-Akt, without affecting p-rpS6. (F) The combination of siRNA directed against Akt3 with AktI-1/2 led to undetectable p-Akt. The abundance of p-rpS6 was unaffected, whereas phosphorylation of the Akt targets p-Gsk3β and p-Tsc2 was decreased. The experiment was performed in 10% FBS. (G) Phosphorylation of rpS6 in LN229:EGFR cells was blocked by both the mTOR inhibitor rapamycin (100 nM) and the EGFR inhibitor erlotinib (5 μM) and unaffected by the PI3K inhibitor PIK-90 (1 μM) or AktI-1/2 (2 μM).