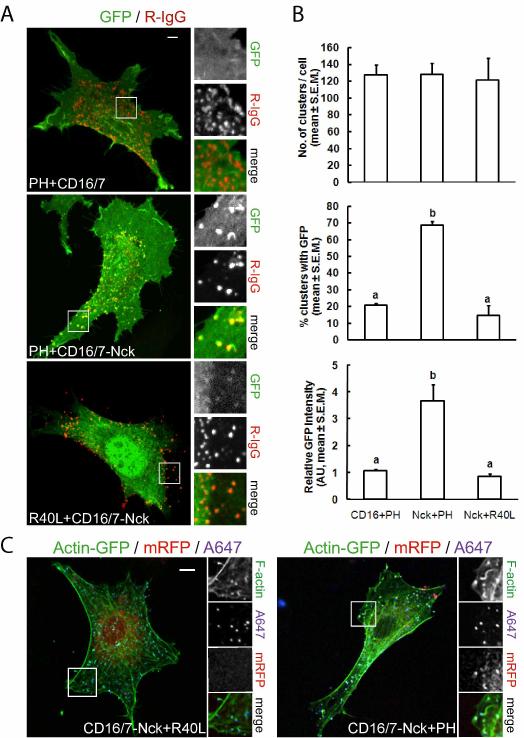
Fig. 3.
Enrichment of PI(4,5)P2 at sites of actin polymerization induced by clusters of membrane-targeted Nck SH3 domains. The pleckstrin homology (PH) domain from PLCδ1 fused with GFP was utilized as a reporter of the subcellular distribution of PI(4,5)P2. A fusion of GFP with a mutant PH domain (PHR40L), unable to bind PI(4,5)P2, was utilized as a control of phosphoinositide binding specificity. A) Subcellular localization of PI(4,5)P2 in NIH3T3 cells expressing the fusion of CD16/7 with the three SH3 domains from Nck (CD16/7-Nck) or CD16/7 alone (CD16/7) after clustering with antibodies (R-IgG). B) Quantitative analysis of the localized enrichment of PI(4,5)P2 at clusters of CD16/7-Nck vs. CD16/7 alone (a vs. b, p<0.05). C) Clustering of CD16/7-Nck induces rearrangements of the actin cytoskeleton, including the formation of actin comets and foci. Colocalization of the wild type variant of the PI(4,5)P2 reporter (PH), but not its inactive mutant (R40L), demonstrates enrichment of PI(4,5)P2 at sites of actin polymerization induced by aggregates of Nck SH3 domains. Scale bars represent 5 μm.