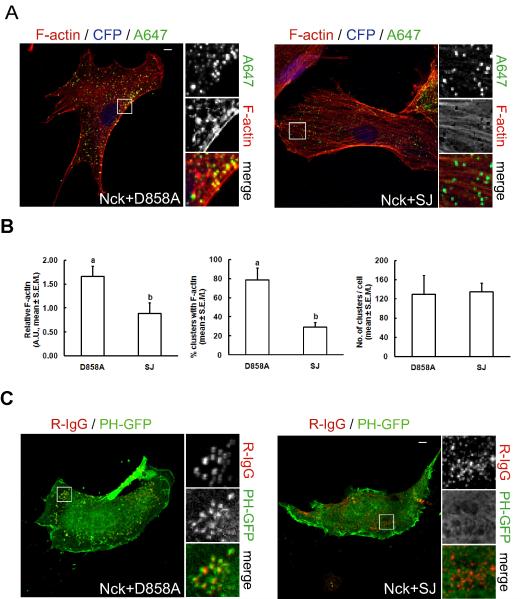
Fig. 6.
Decreased local concentration of PI(4,5)P2 attenuates Nck SH3 domain-induced actin polymerization in living cells. A) Co-aggregation (as illustrated in Fig. 4 B) of membrane-targeted Nck SH3 domains with the wild type PI 5-phosphatase domain of synaptojanin-1 (Nck+SJ), but not with its catalytic inactive mutant (Nck+D858A), attenuates localized actin polymerization. The CD16/7-SJ fusion was expressed in conjunction with CFP from a bicistronic transcript. Clusters of membrane-targeted fusions proteins and F-actin were labeled with Alexa Fluor 647-conjugated IgG (A647) and Texas-red phalloidin, respectively. Scale bar represents 5 μm. B) Computer-assisted, quantitative analysis of phenotypic changes induced by co-aggregation of CD16/7-Nck with membrane-targeted wild type (SJ) or catalytically inactive (D858A) IP 5-phosphatase domain from synaptojanin-1 (a vs. b, p<0.05). Values represent mean ± S.E.M. from images corresponding to 5–7 cells/treatment analyzed in each of three independent experiment (n=3). C) Confocal images of cells co-expressing the PI(4,5)P2 biosensor (PH-GFP), and the combination of CD16/7-Nck with CD16/7-SJ or CD16/7-D858A. Clustering of fusion proteins was performed as described in Fig. 4B. Scale bar represents 5 μm.