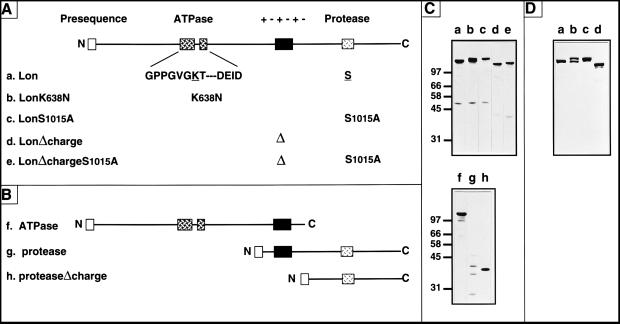
Figure 1.
(A and B) The Lon mutants used in this study. (A) Full-length Lon (a) was mutated in the following ways: LonK638N (b), the ATPase site (Boxes A and B are shown) was mutated by replacing the highly conserved lysine-638 with asparagine; LonS1015A (c), the protease site was inactivated by replacing serine-1015 with alanine; LonΔcharge (d), the charged region of 54 aa (+−+−+−) was deleted; LonΔchargeS1015A (e), deletion of the charged region was combined with replacement of serine-1015 with alanine. (B) Truncated variants of Lon: ATPase (f), a termination codon was introduced after threonine-917; protease (g), the first 75 aa, which include the targeting presequence, were fused in front of glycine-793; proteaseΔcharge (h), the first 75 aa of Lon were fused to glycine-793, thereby deleting the charged region. All truncated constructs carry the mitochondrial-targeting presequence of the authentic Lon precursor. (C) Total cellular proteins extracted from Δlon yeast cells overproducing wild-type Lon (a) or the Lon mutant proteins (b–h) were immunoblotted with antisera recognizing yeast Lon. (D) Coomassie blue-stained gel of purified wild-type Lon, LonK638N, LonS1015A, and LonΔcharge (a–d).