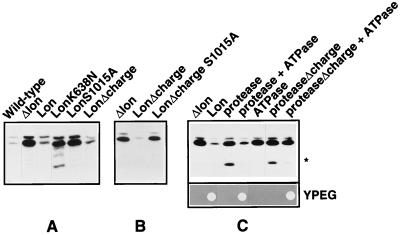
Figure 2.
The ATPase and protease regions of Lon are required for proteolysis and respiration-dependent growth. Proteolytic activity was monitored by the steady-state levels of Mrp20p, a protein of mitochondrial ribosomes; total cell extracts were immunoblotted with a mAb recognizing Mrp20p. (A and B) Wild-type, cells carrying an intact chromosomal copy of the LON gene and the “empty” plasmid pSEYc68 but lacking mtDNA; Δlon, cells whose LON gene had been deleted and that carry the “empty” plasmid pSEYc68; Lon, LonK638N, LonS1015A, LonΔcharge, and LonΔcharge S1015A, Δlon cells carrying plasmid pSEYc68, which contains the corresponding LON gene under the control of the GAL1 promoter. Transformants were grown in selective liquid medium containing Casamino acids and galactose at 30°C for approximately three generations. ∗, A 15-kDa degradation product of Mrp20p. (C) Coexpression of the separated ATPase and protease domains of Lon restores proteolytic activity and complements respiration-dependent growth of Δlon cells. (Upper) The protease, proteaseΔcharge, or ATPase regions of Lon were expressed in Δlon cells from plasmid-borne genes controlled by the GAL1 promoter except for the ATPase region, which was expressed under the authentic LON promoter on a 2-μ plasmid. Transformants were grown as in A and B, except that the selective liquid medium contained a mixture of nonessential amino acids (minus leucine) for plasmid maintenance. (Lower) A diploid yeast strain lacking one chromosomal copy of LON was transformed with two plasmids: a centromere-based plasmid (pSEYc68) carrying the gene for one of the two protease domain constructs of Lon under the control of the GAL1 promoter, and a 2-μ plasmid carrying the gene for the ATPase domain under the control of the authentic LON promoter. Transformants were sporulated and tetrads were dissected on galactose-containing rich medium. The resulting haploid segregants producing the indicated proteins were tested for respiration-dependent growth on yeast extract/peptone/ethanol/glycerol (YPEG).