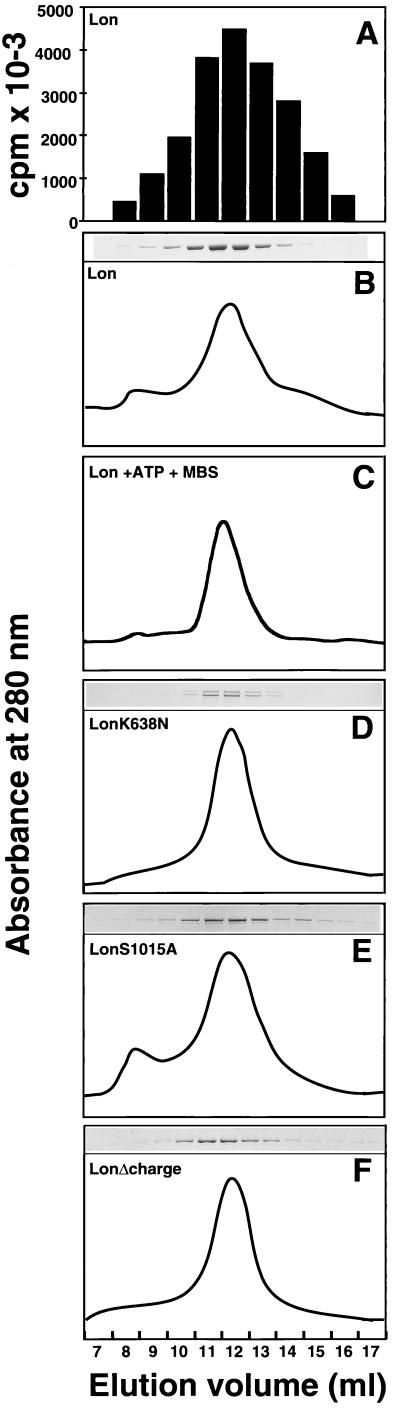
Figure 3.
Oligomeric state of wild-type Lon and its variants determined by gel filtration. Lon, LonK638N, LonS1015A, and LonΔcharge were isolated by binding to Ni2+-agarose, and 60–90 μg of pure protein was analyzed by gel filtration. (A) Purified wild-type Lon from the corresponding fractions shown in B was assayed for ATP-dependent protease activity. (B–F) Absorbance at 280 nm and Coomassie blue-stained protein of eluted fractions. (C) Wild-type Lon was purified as in A, except that 1 mM ATP was added during solubilization and purification on Ni2+-agarose. The isolated protein was crosslinked with 1 mM m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) for 60 min on ice, and 60 μg of protein was analyzed by gel filtration.