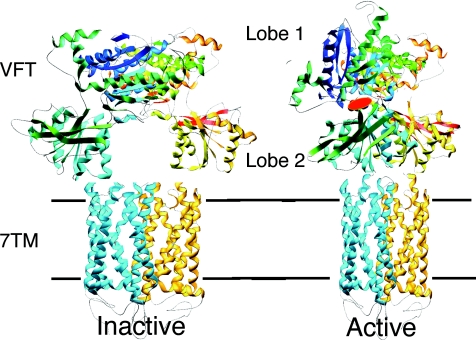
Figure 1. A model for the activation mechanism of the heterodimeric GABAB receptor based on the solved three-dimensional structure of the dimeric extracellular domain of mGlu1.
GABAB1 is in blue, GABAB2 in yellow. Binding of GABA in the cleft of GABAB1 Venus Flytrap domain (VFT) leads to domain closure and a reorientation of both VFTs in the dimer. This is expected to induce a relative movement of the seven transmembrane (7TM) domains, allowing activation of GABAB2 7TM. A similar activation mechanism is proposed for the mGlu receptors even though these have an additional cystein-rich domain that links their VFT to their 7TM.