Short abstract
An outline of broad principles that should be considered when integrating an electronic health record, and in particular, a chemotherapy ordering module, into practice.
This is the first of a two-part review, concentrating on the ability of oncology electronic health records (EHRs) to enhance patient safety through the chemotherapy ordering and administration process, and on standardization of workflow processes in the practice. In this article, we endeavor to outline broad principles that should be considered when integrating an EHR, and in particular, a chemotherapy ordering module, into practice. We strongly advocate attention to these principles, as any fundamental change in a drug ordering process may compromise safeguards that are present in the practice.
Computerized Order Entry and Workflow Policy
EHRs are being adopted with increasing frequency. They bring efficiencies to practice record keeping and billing. They allow data to be accessed for multiple purposes by different providers and employees of a practice, thereby cutting down on inefficiencies created by relying on one paper record. A true EHR will collect patient data, integrate this information with data from other sources, and guide the provider with clinical decision support in real-time care of a patient. EHRs can also provide data for multiple purposes such as for analysis of practice demographics and reporting on quality measures.
EHRs that support oncologists must take into account key areas of practice that differentiate oncology from other specialties. Accurate tumor staging, flow sheets, the need for multidisciplinary workflow documentation, integration of laboratory and imaging reporting, and dealing with chemotherapy ordering and toxicities are some of these unique demands.1 Particularly demanding is the ordering, documentation, and management of chemotherapy and ancillary medications.
Regrettably, medication errors related to chemotherapy from the use of paper-based records and manual systems are not uncommon, and have been the source of some notorious cases of patient harm in recent years. Even when computerized order entry systems are used, errors are still possible because of human error in the process or due to inherent properties of the computerized system. Data collected from three different outpatient infusion centers at the Dana-Farber Cancer Institute in Boston in the year 2000, using a first-generation computerized order entry system, showed a medication error rate of 3% in adult patients (249 errors of 8,008 medication orders reviewed). Of these, more than one third were related to chemotherapy, which constituted 4% of all adult chemotherapy orders written during the interval studied.2 Of the potential adverse drug events identified, 26% were serious, including such things as missed orders for premedications and overlooked chemotherapy treatment parameters such as a low WBC count.
Workflow is the source of much of the risk to patients during a typical visit to the clinic for chemotherapy. In the typical treatment day, the patient proceeds from laboratory to physician visit to the infusion suite for chemotherapy, and each step involves numerous manual processes and hand-offs that are subject to error.3 For example, although it is recognized that treatment should be selected on the basis of established guidelines and/or best practices, there is no single authoritative source for chemotherapy regimens, and consequently many oncologists create customized order sets of commonly used regimens. They refer to these compilations multiple times during the workday, so that chemotherapy is typically ordered on an individual basis and is subject to errors involving misreading or misapplying source material and computational errors. Hand-offs between physicians and nurses or physicians and pharmacists/admixture technicians can be affected by misinterpretation of physician handwriting and miscommunication involving verbal orders or other assumptions. Oncology EHRs can attenuate some of these risks by forcing structure to the workflow and by ensuring complete and accurate data to be available at each step of the process. Regimentation and standardization addresses the ad hoc components in the clinic that can contribute to error.
Chemotherapy ordering, preparation, and administration remain high-risk procedures in oncology practice. Many antineoplastic agents have a narrow therapeutic index. Inappropriately low doses will result in loss of efficacy and inappropriately high doses can lead to unnecessary toxicity and possibly death. In addition, these agents are frequently dosed based on patient height, weight, serum creatinine, age, and so on, and the incorporation of these data usually involves complex calculations. Computerized order entry systems can ease the ordering process and improve safety. Though systems are being developed with these goals in mind, there is little in the literature to suggest principles that should underlie the creation of these systems. We have lacked guidance about how physician, nurse, and pharmacist workflow should be designed and practice policies instituted to support patient safety when these systems are in use. Chemotherapy ordering, preparation, and administration occur in both ambulatory and inpatient settings, which present special challenges. Often other health professionals such as fellows, housestaff, nurse practitioners, and physician assistants are brought into the process, further complicating matters. Computerized chemotherapy order entry systems may also be stand-alone products or be integrated or linked with electronic medical records, scheduling systems, and billing systems.
Most of the principles and practice policy that have been developed and incorporated into systems have arisen from lessons learned from medication errors. Anecdotal literature exists which outlines instances of errors and subsequent root cause analyses leading to the development of safety principles and practice. Few systematic studies exist to address these issues. Randomized clinical trials are not likely to be performed to test the safety performance of computerized order entry systems and their specific features, or the safety policies concerning practice of physicians, nurses, and pharmacists in this area. Studies of medication errors in general medical units have been performed before and after implementation of computerized order entry systems, and have shown a dramatic decrease in the frequency of medication errors.4,5 Principles behind these systems and workflow policy are likely to be based on what makes sense as well as lessons learned from known chemotherapy errors. Learning can also occur from “near misses”—episodes where an error was made somewhere in the system, but identified and corrected before the error reached the patient. These are ideal learning opportunities, given that no harm was done, and corrective action can lead to improved systems and policies, thus reducing the chance for future errors that might result in patient harm.
With current technology, no matter how good a computerized system is, it does not remove the need for human action and therefore the possibility of human error. A human must enter orders into a system. People, not things, make mistakes.6 Toxicities must be accurately assessed, and laboratory values taken into account. Nurses and/or pharmacists must review orders verifying their accuracy, then prepare the agents. Nurses then must administer the right agents at the right dose, via the right route, in the correct manner, and to the correct patient. Degrees of automation can reduce human actions and thereby reduce the chances for error. For instance, a system can take into account a laboratory test result such as the absolute neutrophil count, and automatically use this information in the ordering system to modify a chemotherapy dose. The challenge is to design a computerized system and integrate it into a practice so that it works in a way that minimizes chance for error.
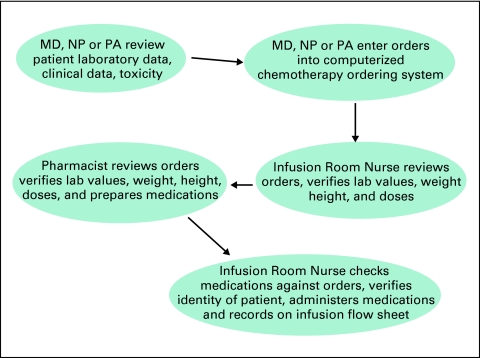
Workflow and clinical policies will differ from institution to institution, and practice to practice, but in a general sense might reflect that outlined in Figure 1
Figure 1.
An Example of Oncology Workflow in Regard to Chemotherapy Ordering, Preparation and Administration
We believe that the principles outlined below should underlie the functioning of a computerized chemotherapy order entry system, as well as policies that govern the physician, nurse, and pharmacist workflow. The transformation of the patient's flow in the clinic will be driven by the intersection of the functionality of the EHR and the change in workflow demanded by these principles. These are meant as general guides and may vary in different practice situations, but the overarching principle is that of safety and effectiveness of these procedures.
Principles of a Computerized Chemotherapy Order Entry System
Purpose
It is a system developed and used by health care providers to effectively, safely, and efficiently order anticancer agents and associated ancillary therapies.
General Principles
Accuracy.
Orders reflect the intent of the ordering clinician, and the independent understanding, confirmation, and approval of the nurse and pharmacist. No verbal or written (nonelectronic) orders are accepted. This helps to ensure the intent of the ordering clinician is clearly documented and followed. Any verbal misunderstandings, confusion due to poor legibility, or other sources of error are eliminated.
Standardization.
All aspects of the ordering process are standardized, to the extent that it is feasible and reasonable to do so. This includes chemotherapy regimens, associated supportive therapies (eg, antiemetics, hydration, and so on), and treatments for hypersensitivity reactions. Standardization improves familiarity with the specifics of order sets, the criteria for use and details, and ultimately the safety of orders. If a regimen such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is given in several doses, schedules, and administration schemes, nurses, pharmacists, and others will likely be less certain of the specifics of a regimen, and may wonder, “Is this the R-CHOP Dr Jones orders, or the one Dr Smith orders?” Studies in anesthesia and other industries have demonstrated the increased risk of error due to lack of standardization.7
Chemotherapy regimens are based on published trials or abstracts and/or expert consensus, with known dosing and schedule parameters as well as expected toxicities. Supportive therapies are included in the chemotherapy order set and are derived from guidelines (American Society of Hematology, ASCO, National Comprehensive Cancer Network, and so on). A practice setting should have a formalized process in place to review these guidelines and prioritize their implementation into the EHR. The system will default to these guidelines, though there will be flexibility to tailor antiemetic and other supportive therapy to the needs of the individual patient.
Automation.
Whenever possible, calculations are performed automatically by the computer system to reduce clinician workload and avoid errors.8 This includes calculation of body-surface area, per kilogram dosing, and area under the curve formulas. Ideally these parameters would be drawn automatically from other sections of the EHR such as the vital signs, flow sheets, and laboratory data.
Decision support.
The system contains embedded tools that allow for computerized decision support, including dose ranges, maximum dose thresholds that cannot be exceeded, allergy alerts, and suggested dose reductions.9 For example, when a chemotherapy regimen causes a particular toxicity, the system will offer suggested dose reductions by percentage (eg, 20% based on blood counts, bilirubin, and so on), and when selected, the calculations for these dose reductions are also performed automatically. An interdisciplinary team of physicians, nurses, and pharmacists propose and approve each decision support element.
Flexibility.
The system can be modified as current treatments change and new treatments are developed. Orders for chemotherapy regimens are divided into different folders, so changes can be made to one folder (eg, the antiemetics) without affecting the other folders (eg, the chemotherapy medications). Orders for investigational protocols are also included within the ordering system, and can be modified if protocols are amended.
Workflow integration.
The system is designed to be an integrated element of the interdisciplinary process of ordering and administering chemotherapy. As such, the system facilitates the principle of shared responsibility (where ordering clinicians, infusion room nurses, and pharmacists share the responsibility that orders are correct), redundancy (where the system decreases the likelihood that errors will reach the patient), and minimization of ambiguity (where the system helps ensure that orders reflect the intention of the ordering provider).
Safety over convenience.
When decisions are made concerning the design and functionality of the system, safety concerns take precedence over convenience. In this process, workflow is always taken into consideration.
Efficiency, reliability, and usability.
Orders can be entered and are communicated to pharmacy and support staff in the same or less time than if done on paper. The system is available whenever and wherever needed (ie, there is minimal unscheduled down time). The system is designed to perform in a logical and straightforward manner, be user friendly, and available to users while they are off site via a virtual private network or similar functionality.
Implementation
To institute these principles, the practice will need to formalize a governance structure to address the many decisions demanded by the system and the changes in workflow. In large institutions, existent committees, such as a pharmacy and therapeutics committee or a patient care committee, may be vested with these responsibilities. Smaller practices will need to create this process. The committee can be small but must be multidisciplinary and completely engaged in the process. These committees should both standardize regimens with respect to antineoplastic agents and ancillary medications, and ensure all orders are supported by credible literature. In addition, the committees should analyze errors and “near misses” which occur in the practice in order to alter systems or workflow to reduce the likelihood of errors occurring in the future.
In the September 2008 issue of the Journal of Oncology Practice, we will review operational details that are mandated by the application of these principles. We hope that these two articles will provide practices a rationale to choose and implement an oncology EHR in such a manner to take advantage of the improvements in patient safety they can offer.
ASCO is committed to providing oncologists with tools to enhance the safety of patients and assist the oncologist in providing quality cancer care. EHRs have the promise of transforming our practice. ASCO offers an extensive review of the steps and pitfalls of choosing and implementing an electronic health record in a recently published field guide. The Oncology Electronic Health Record Field Guide: Selecting and Implementing an EHR, is available through ASCO (www.asco.org/ehrfieldguide).
ASCO members will also find additional resources, including links to online virtual meeting presentations from the ASCO EHR Symposium (www.asco.org/ehr) and to selected articles published in JOP.
Acknowledgment
All authors are members of the Electronic Health Records Workgroup of ASCO.
References
- 1.American Society of Clinical Oncology: The Oncologist's Field Guide to Selecting and Implementing an Electronic Health Record, http://www.asco.org/ehrfieldguide, Alexandria, VA, ASCO, 2008
- 2.Gandhi TK, Bartel SB, Shulman LN, et al: Medication safety in the ambulatory chemotherapy setting. Cancer 104:2477-2483, 2005 [DOI] [PubMed] [Google Scholar]
- 3.American Society of Clinical Oncology: Ensuring continuity of care for patients with cancer through electronic health records: Recommendations from ASCO's 2007 EHR Roundtable. http://www.asco.org/ASCO/Practice+Resources/Electronic+Health+Records/EHR+Events/Electronic+Health+Records+Roundtable?cpsextcurrchannel=1 [DOI] [PMC free article] [PubMed]
- 4.Bates DW, Gawande AA: Improved safety with information technology. N Engl J Med 348:25, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Voeffray M, Pannatier A, Stupp R, et al: Effect of computerisation on the quality and safety of chemotherapy prescription. Qual Saf Health Care 15:418-421, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonks A: Safer by design. BMJ 336:186-188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachter R, McDonald K, Shojania K, et al: Making health care safer: A critical analysis of patient safety practices. Evidence/Technology Report for AHRQ. http://www.ahrq.gov/clinic/ptsafety/pdf/ptsafety.pdf [PMC free article] [PubMed]
- 8.DuBeshter B, Walsh CJ, Altobelli K, et al: Experience with computerized chemotherapy order entry. J Oncol Pract 2:49-52, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuBeshter B, Griggs J, Angel C, et al: Chemotherapy dose limits set by users of a computer order entry system. Hosp Pharm 41:136-142, 2006 [Google Scholar]