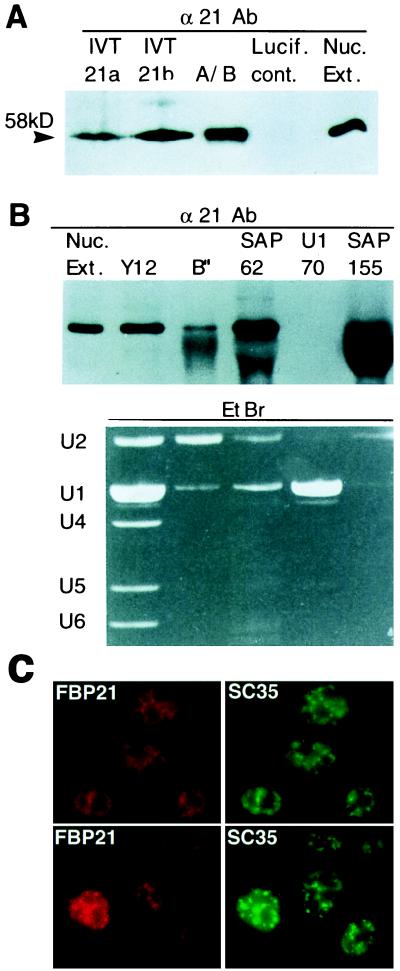
Figure 4.
FBP21 is a 58-kDa protein that is associated with the U2 snRNP. (A) Analysis of in vitro-translated FBP21. Two different cDNAs (21a and 21b) for FBP21 were transcribed and translated in vitro in reticulocyte lysates as described (Promega). Translated products were run on a SDS/PAGE gel with an A/B complex of spliceosome-specific proteins (A/B), a luciferase in vitro translation product as a control (Lucif. cont.), and HeLa cell nuclear extract (Nuc. Ext.) and were blotted to nitrocellulose. The filter was probed with the α21Ab antibody. The arrowhead shows the detected band of 58 kDa. (B) Association between FBP21 and U2 snRNP’s. The indicated antibodies (Y12, anti-SmB, and SmB′; B", anti-B"; SAP 62, anti-SAP 62; U1–70, anti-U1–70, and SAP 155, anti-SAP 155) were used to perform immunoprecipitations of HeLa cell nuclear extracts under RNase free conditions. The immunoprecipitations were washed three times in a buffer containing 250 mM NaCl and 50 mM Tris (pH8). The immunoprecipitations then were divided in two. One half was run on a SDS/PAGE gel, was blotted to nitrocellulose, and was probed with α21Ab (Upper). The other half of the immunoprecipitation also was fractionated on a SDS/PAGE gel and was stained with EtBr to detect snRNAs (Lower). The large protein band seen in the SAP 155 lane is Ig heavy chain. A light band corresponding to FBP21 can be seen above it. (C) Localization of endogenous FBP21. HeLa cells were fixed, and the cellular localization of FBP21 was determined by indirect immunofluorescence microscopy with αFBP21 antibodies. The FBP21 protein is localized in a speckled nuclear pattern, characteristic of splicing factors. FBP21 colocalizes with SC35, an SR protein that is required for pre-mRNA splicing and is a characterized marker for nuclear speckles. Two different fields are shown. The Texas Red signal represents the endogenous FBP21 protein, and the FITC signal represents SC35 SR protein.