Abstract
Maternally inherited Wolbachia bacteria have evolved mechanisms to manipulate the reproduction of their invertebrate hosts, promoting infection spread. A high fitness cost to the host is maladaptive for obligate endosymbionts, and prior studies show rapid selection of new Wolbachia associations toward commensal or mutualistic symbioses. Here, wMelPop Wolbachia is transferred from Drosophila melanogaster into the mosquito Aedes albopictus. Characterization of the resulting strain provides an extreme example of Wolbachia as a pathogen. In addition to reduced longevity and fecundity, abnormally high Wolbachia density is associated with embryonic mortality that masks the typical pattern of cytoplasmic incompatibility. The results are consistent with earlier reports that show unpredictable shifts in the Wolbachia phenotype after interspecific transfer, which can complicate proposed strategies to modify the age structure of medically important vector populations.
Wolbachia bacteria have been identified within a diverse array of invertebrates, where infections are responsible for a variety of host effects including male killing, parthenogenesis, feminization and cytoplasmic incompatibility (CI) (29). The CI phenotype is characterized by early embryonic arrest and a reduction in the number of viable progeny (7, 8, 18, 39). Strict maternal inheritance via embryonic cytoplasm is observed with Wolbachia, and although Wolbachia numbers can be high in testes (24), transmission of the infection to offspring via males has not been reported (17, 41). Instead, an unidentified “modification” of sperm acts to initiate CI in fertilized embryos, unless “rescued” by a compatible Wolbachia infection in their mates (8). The cost of CI to hosts falls upon uninfected females and infected males within the host population, and since males are a dead-end for Wolbachia infection, the resulting dynamics can lead to the spread of infection above an unstable equilibrium threshold (18).
Wolbachia bacteria are generally described as “reproductive parasites,” and Wolbachia-host interactions include examples that span the symbiosis spectrum. Field and laboratory studies support hypothesized trends from pathogenicity toward commensalisms and/or mutualism (16, 25, 40). Since mutualistic examples are hypothesized to represent older associations, it follows that maladapted symbioses will be more common among new associations, including artificially generated infections. It is surprising, therefore, that additional examples of pathogenic Wolbachia symbioses have not been identified to date, especially given examples of Wolbachia transinfection. To date, there are two reported examples of pathogenic Wolbachia: an artificially generated association between the isopod Porcellio dilatatus and Wolbachia injected from Armadillium (2) and the wMelPop Wolbachia infection in Drosophila (27). Both examples are similar in that host mortality occurs relatively late and is associated with Wolbachia overproliferation in adult tissues (22, 27). A prior artificial transfer of the wMelPop infection into D. simulans led to a transient exaggeration of pathogenic effects, which were ameliorated in later generations (24, 25).
A recent report of the stable introduction of wMelPop into the medically important mosquito disease vector Aedes aegypti (26) suggests a potential strategy to control disease transmission utilizing the heritable Wolbachia infection. Since female mosquitoes must survive an extrinsic incubation period to transmit dengue or other pathogens, a Wolbachia-induced shift in the population age structure toward younger females is expected to reduce pathogen transmission (6, 9).
Aedes albopictus (Asian tiger mosquito) is a globally invasive mosquito that has spread via accidental human transport and competitive dominance, resulting in its displacement of numerous resident mosquito populations (15, 30, 31). Its relevance as a disease vector has been elevated recently due to its role in recent chikungunya outbreaks (1, 14, 21, 36).
Populations of A. albopictus are normally superinfected with two Wolbachia strains: wAlbA and wAlbB (23, 37). The infection is among the most mutualistic of associations described for Wolbachia in insects (12). Here, we introduced wMelPop into A. albopictus as the first step toward modifying age structure of an A. albopictus population in order to decrease disease transmission such as dengue. However, the wMelPop infection in A. albopictus was maladaptive and provided an extreme example of Wolbachia as a pathogen.
MATERIALS AND METHODS
Insect strains.
Experiments were conducted using the wMelPop-infected colony of Drosophila melanogaster (w1118) (27), naturally superinfected A. albopictus (Hou and IH strains) (12, 37), and an aposymbiotic (i.e., uninfected) A. albopictus strain (HT1) (11). Drosophila and mosquito strains were maintained as described previously (10, 34).
Microinjection.
Injection techniques for embryonic transinfection of mosquito and Drosophila were as previously described (42). Injection needles were pulled from quartz microcapillaries (QF 100-70-7.5; Sutter Instrument Co., Novato, CA) by using a P2000 (Sutter Instrument Co., Novato, CA). wMelPop-infected cytoplasm was withdrawn from the posterior pole of donor w1118 embryos and injected into the posterior pole of HT1 embryos by using an IM300 microinjector (Narishige Scientific, Tokyo, Japan). Injected embryos were transferred onto wet filter paper, incubated at 27 ± 2°C and 75 ± 10% relative humidity for 5 days, and then submerged in deoxygenated water. Resulting larvae (G0) were reared using standard conditions (10), and pupae were isolated as virgins. Eclosing females were mated with HT1 males (i.e., uninfected), blood fed, allowed to oviposit, and then PCR assayed to determine their Wolbachia infection status. Females failing to produce eggs were not tested.
PCR amplification and fluorescence in situ hybridization.
Two primer sets were used to confirm wMelPop infection status: wMelPop-specific primers (VNTR141F/141R) (33) and general Wolbachia wsp primers (81F/691R) (44). DNA was extracted from adult mosquitoes as described previously (5). PCR amplification was performed in 20-μl reaction volumes using Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. An MJ Research PTC-200 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA) was used to perform 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Template quality was confirmed in samples failing to amplify Wolbachia DNA by using 12S mitochondrial primers as previously described (28). Fluorescence in situ hybridization staining was performed according to the method of Xi et al. (41).
HTM curing.
Wolbachia was removed from G10 HTM using tetracycline according to methods described previously (11). The absence of Wolbachia in the resulting HTMT line was confirmed by PCR (10 females and 10 males) for two consecutive generations after tetracycline treatment. To minimize the potential for direct tetracycline effects, crosses with HTMT individuals were performed two generations after tetracycline treatment.
Longevity, CI, and fecundity assays.
For longevity assays, G5 and G13 eggs were hatched, and the resulting larvae were reared under optimal rearing conditions (i.e., low larval density and liver powder provided ad libitum). Newly emerged adults (10 females and 10 males) were placed in cages (n = 20) and provided with a constant 10% sucrose solution. An anesthetized mouse was provided weekly for blood feeding (A3336-01; PHS Assurance). An oviposition cup lined with wet paper was continuously available, with weekly exchanges. Dead mosquitoes were collected at 12-h intervals, and sex was determined until all individuals in a cage died. The longevity of females and males was compared separately by using a Kaplan-Meier log-rank test (JMP 7.0.2; SAS Institute, Cary, NC).
For the CI and fecundity assays, G13 eggs were used. Rearing of larvae and adults, blood feeding, and egg collection were performed as described above. Eggs from the first batch were hatched after 5 days of maturation, and the resulting egg number and arcsine transformed hatch rates were compared by using analysis of variance (ANOVA) with post hoc Tukey honestly significant difference (HSD) at P < 0.05 (SPSS 11.5; SAS Institute).
To assess the effects of time (gonotrophic cycle) and parental types used in crosses, repeated-measures ANOVA tests were performed (SPSS 11.5). If the Mauchly's test indicated violation of the sphericity assumption, the degrees of freedom were corrected by using Huynh-Feldt estimates. Multiple comparisons between cross types used post hoc Tukey HSD.
To confirm insemination, spermathecae were checked in a subset of females by dissecting in Ringer's solution and observing under a dissecting microscope for sperm. Embryonic development was characterized by attaching eggs to double-sided tape (Scotch 665; 3M, St. Paul, MN) on a slide glass in a drop of Clorox bleach (Clorox, Oakland, CA) for 30 min, observed by using an Olympus IX70 fluorescence microscope, and photographed using Magnafire software (Optronics, Goleta, CA). The relative proportions of developmental stages for the three groups were compared among cross types by using ANOVA with post hoc Tukey HSD at P < 0.05.
RESULTS
Cytoplasm from wMelPop-infected Drosophila embryos was injected into aposymbiotic A. albopictus embryos (HT1 strain), resulting in 13 females (G0) from five microinjection experiments, 8 of which were infected and produced progeny, allowing the establishment of isofemale lines (Table 1). The “HTM” isofemale line was selected for additional characterization, based upon the relative stability of maternal inheritance. At G10, the HTM line was subdivided and one of the resulting lines was closed (i.e., females no longer outcrossed with HT1 males but mated with HTM males). Outcrossing with HT1 males continued in the other line.
TABLE 1.
Survival and infection status of A. albopictus microinjected with wMelPop Wolbachiaa
| Expt | % Hatch (larvae/injected eggs) | % Pupation (pupae/larvae) | % Eclosion (adults/pupae) | G0 infection status (% infected) |
|
|---|---|---|---|---|---|
| Female (infected female/total tested) | Male (infected male/total tested) | ||||
| 1 | 17.5 (17/97) | 94.1 (16/17) | 100.0 (16/16) | 66.7 (4/6) | 75.0 (6/8) |
| 2 | 5.2 (7/134) | 100.0 (7/7) | 71.4 (5/7) | NAb (0/0) | 50.0 (1/2) |
| 3 | 15.7 (21/134) | 90.5 (19/21) | 100.0 (19/19) | 60.0 (3/5) | 55.6 (5/9) |
| 4 | 4.8 (6/126) | 83.3 (5/6) | 60.0 (3/5) | NA (0/0) | 100.0 (2/2) |
| 5 | 5.5 (6/110) | 100.0 (6/6) | 100.0 (6/6) | 50.0 (1/2) | 100.0 (3/3) |
The numbers of larvae, eggs, pupae, etc., are presented in parentheses as indicated.
NA, not applicable.
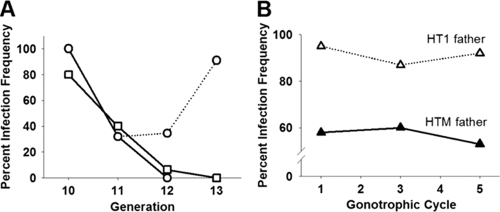
In the outcrossed HTM line, high maternal inheritance continued through G10, with 99.8% ± 0.13% (mean ± standard error [SE]; n = 150) and 100% (n = 38) infection detected in females and males, respectively. In contrast, the infection frequency dropped in the closed line, resulting in the loss of Wolbachia infection within three generations in the absence of selection for wMelPop infection (Fig. 1A). In a second experiment, closing of the HTM line was associated with a similar decline in infection frequency at G11 without selection for wMelPop infection (Fig. 1A). The line was split at G11, and females were either mated by HTM or HT1 males and selected for wMelPop infection. Although both closed and outcrossed lines were selected for wMelPop infection, only the outcrossed line subsequently restored infection up to 91% at G13, and the closed line lost the infection (Fig. 1A). As an additional test for paternal effects on Wolbachia infection frequency, G13 HTM females were mated with either HT1 or HTM males. PCR assays of the resulting progeny demonstrated 91.3% ± 2.7% and 57.0% ± 2.4% (mean ± the SE) infection frequencies, respectively (Fig. 1B). The absence of paternal transmission of the wMelPop infection to progeny was confirmed by PCR assays of 60 first-instar larvae resulting from three gonotrophic cycles of HT1 females crossed with HTM males, which resulted in no infection in progenies.
FIG. 1.
Infection frequency among progeny of HTM females mated with either HTM males (solid line) or HT1 males (broken line). (A) Experiment I (□), closed HTM line with no selection; experiment II (○), closed and outcrossed line selecting for infection at G11. (B) Experiment III, closed and outcrossed line at G13 across five gonotrophic cycles. Closing the population resulted in loss of infection in experiments, whereas outcrossing restored infection.
The egg hatch rates resulting from the four cross combinations between HTM and HT1 individuals were examined (Fig. 2). Significantly reduced hatch rates (multiple comparisons with post hoc Tukey HSD, P < 0.0001) were observed in all three cross types that included HTM individuals (female or male) relative to crosses between uninfected individuals. No difference in egg hatch was observed between crosses that included HTM individuals. The egg hatch rates remained consistent across multiple gonotrophic cycles (repeated measures ANOVA, Huynh-Feldt correction; F = 2.6, df = 3.16, P = 0.060). To examine the possibility that reduced egg hatch resulted from failure of HTM males to inseminate females, spermathecae were examined from females in each cross type (n = 25), and sperm were observed in 100% of the spermathecae.
FIG. 2.
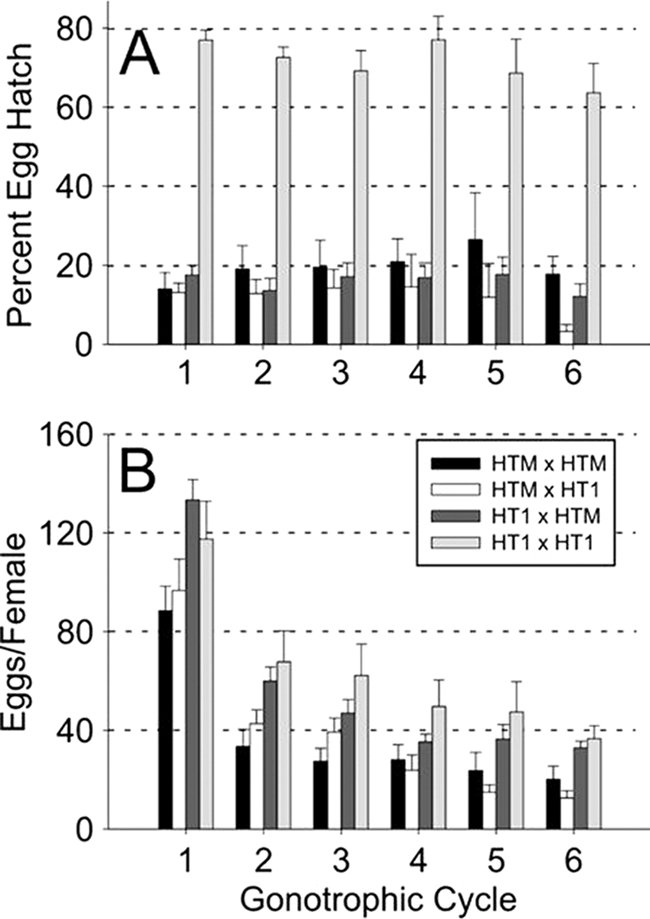
Percent egg hatch (A) and egg number (B) resulting from crosses between HTM and HT1 A. albopictus strains infected with wMelPop Wolbachia and uninfected, respectively. The data are shown for six gonotrophic cycles. Crosses are shown as female × male. Error bars indicate the SE. Unlike the typical CI phenotype, wMelPop resulted in low hatch rates from cross types expected to be compatible (i.e., HTM × HTM and HTM × HT1).
Although female fecundity was observed to decrease significantly across gonotrophic cycles in all four cross types (repeated measures ANOVA, Huynh-Feldt correction; F = 124.1, df = 4.58, P < 0.0001), a comparison of fecundity between cross types revealed a difference between the HTM × HTM (female × male) and HT1 × HT1 cross only (P = 0.045) (Fig. 2). Removal of the wMelPop infection from the HTM line via tetracycline treatment had no effect on fecundity. The fecundity of the cured HTMT line did not differ significantly from HT1 crosses. In contrast, removal of the Wolbachia infection in the HTMT line restored egg hatch to levels indistinguishable from the compatible HT1 crosses (Table 2).
TABLE 2.
Fecundity and hatch rate resulting from crosses of the HTMT straina
| Cross |
Mean ± SE (n) |
||
|---|---|---|---|
| Female | Male | No. of eggs | % Hatch |
| HTMT | HT1 | 840 ± 143 (5) A | 88.8 ± 1.4 (5) B |
| HT1 | HTMT | 597 ± 78 (5) A | 65.3 ± 9.6 (5) C |
| HTMT | HTMT | 646 ± 77 (5) A | 80.0 ± 2.5 (5) BC |
| HT1 | HT1 | 687 ± 54 (5) A | 83.1 ± 2.7 (5) BC |
| HTM | HTM | 541 ± 100 (3) A | 30.4 ± 3.2 (3) D |
The fecundity (i.e., the number of eggs) and hatch rate (i.e., the percent egg hatch) resulting from crosses of the HTMT strain were determined. Different capital letters indicate significant differences (P < 0.05 [ANOVA]).
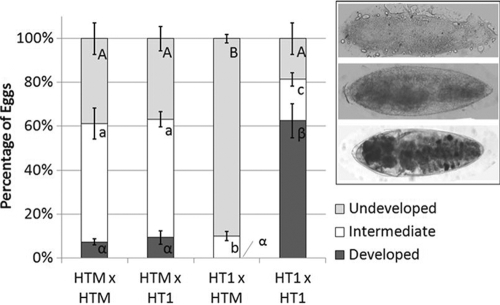
Although egg hatch failure can result from multiple reasons, the typical CI phenotype is characterized by early embryonic arrest (39). Embryonic bleaching was used to examine the development of eggs failing to hatch from HTM crosses. Unhatched eggs were assigned to one of three categories: no development, intermediate development, and visible eye spot (Fig. 3). A majority of unhatching embryos from HT1 × HT1 crosses showed late-stage development (i.e., eye spots). In contrast, the HT1 × HTM cross predominantly resulted in no development among hatching eggs. Both cross types that included HTM females were similar, resulting in proportionally more eggs displaying intermediate levels of development. For the HTM larvae from the outcrossed line that successfully hatch, high survivorship was observed (i.e., similar to naturally infected mosquitoes) (20): 258 pupae/284 larvae (91%) survived to pupate and 94% of the resulting pupae emerged as adults (49% females).
FIG. 3.
Characterization of embryonic development in unhatched embryos resulting from the four possible cross types between the HTM and HT1 strains of A. albopictus, which are infected with wMelPop Wolbachia and uninfected, respectively. Bleached embryos were assigned to three developmental categories: undeveloped, intermediate, and developed (inset, top to bottom). Different letters correspond to significant differences within each embryonic category (P < 0.05). Crosses are shown as female × male. Error bars indicate the SE.
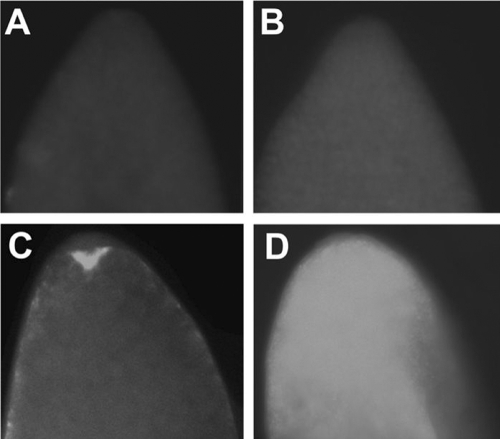
Fluorescence in situ hybridization was used to visualize Wolbachia in the HTM, HT1, HTMT, and IH (i.e., naturally infected) embryos (Fig. 4). HT1 and HTMT were similar in that no Wolbachia were observed. Naturally infected embryos displayed a pattern of Wolbachia infection focused in the periphery and poles, similar to prior descriptions (41). In contrast, the wMelPop infection was higher in HTM embryos and distributed throughout the embryo.
FIG. 4.
Fluorescence in situ hybridization was used to visualize Wolbachia distribution in A. albopictus oocytes from the following strains: HT1, aposymbiotic (A); HTMT, tetracycline-cured HTM (B); IH, naturally superinfected strain (C); and HTM, wMelPop-transinfected (D). A high density of Wolbachia is observed in HTM embryos infected with wMelPolp, and the infection is more broadly distributed relative to that observed in naturally infected embryos.
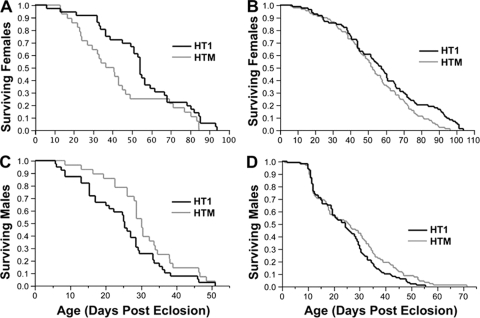
An initial comparison of HTM and HT1 longevity was conducted at G5, showing reduced longevity of HTM females relative to HT1 females (Kaplan-Meier log-rank; χ2 = 4.622, df = 1, P = 0.032) (Fig. 5). The median longevities of HTM and HT1 females were 41 and 54 days, respectively. No difference was observed between HTM and HT1 males, with median ages of 30 and 25 days, respectively (χ2 = 3.286, df = 1, P = 0.070). Repeating the longevity assay at G13 (Fig. 5), HTM females again were observed to be significantly shorter-lived (median age of 52.5 days) relative to HT1 females (median age of 57.5 days) (χ2 = 5.298, df = 1, P = 0.0213). Similar to the G5 longevity assay, no difference was observed between HTM and HT1 males, with median ages of 26 and 24.5 days, respectively (χ2 = 3.0377, df = 1, P = 0.0814).
FIG. 5.
Longevity of A. albopictus females (A and B) and males (C and D) that are either infected with the wMelPop (HTM strain) or are uninfected (HT1 strain) at generations 5 (A and C) and 13 (B and D). HTM infection resulted in a reduced life span for infected females but not for males.
DISCUSSION
The results presented here demonstrate that although A. albopictus is permissive to the wMelPop Wolbachia type, the resulting infection can be best categorized as a pathogenic symbiosis. The results of microinjection experiments are similar to prior transinfection experiments (25, 32), showing that A. albopictus susceptibility to wMelPop is not atypical. However, the resulting phenotype of the HTM strain is unlike other Wolbachia infections and the most pathogenic wMelPop association reported to date. The reduced fitness is due primarily to the low egg hatch of HTM females, which results regardless of male infection status. Maladaptation of wMelPop was pronounced in A. albopictus compared to the results of establishing wMelPop in naturally uninfected A. aegypti (26). Examination of unhatched HTM embryos reveals a high proportion at an intermediate level of development. Late-arrested embryos have been described for CI induction in Culex pipiens when both females and males are Wolbachia infected, suggesting that an infection in females may facilitate limited morphogenesis (13). There are reports also of “suicide” Wolbachia infections, capable of modifying but not rescuing (43). Although partial rescue can potentially explain events resulting in crosses between HTM individuals, it does not explain the low egg hatch resulting from crosses of HTM females and uninfected males, which in theory would not induce CI. Removal of the wMelPop infection (i.e., antibiotic clearing of the HTMT strain) restored normal egg hatch, demonstrating that the low-egg-hatch phenotype is not explained by the HTM genotype or mitotype, which possibly has been changed during microinjection of wMelPop.
Examination of HTM oocytes shows an unusually high density of Wolbachia, suggesting that Wolbachia over-replication in oocytes is responsible for the low egg hatch resulting from HTM females. This is similar to a reported transfer of wMelPop to D. simulans in which the wMelPop infection was associated with a drop in egg hatch (25). In the prior experiment, however, the egg hatch reduction was relatively small in D. simulans and was transient, returning to normal egg hatch levels after five generations of transinfection experiments. Similar to results in transinfected D. simulans (25), lower fecundity was observed in HTM females, consistent with higher costs associated with wMelPop infection.
Wolbachia has been shown to change phenotype upon transfer between hosts (4, 35, 43). With the wMelPop infection in Drosophila, a range of CI penetrance has been described (25, 27, 32). Although CI induction by wMelPop in A. albopictus was not initially apparent, based upon the pattern of egg hatch, subsequent examination of embryonic development reveals a cross pattern that is consistent with CI. Specifically, the cross between uninfected females and HTM males is different from the remaining cross types, resulting in significantly more embryos that are arrested in early development, which is diagnostic of CI. However, the CI is incomplete (∼20% hatch) and relatively weak compared to that resulting in crosses of the natural superinfection (<1% hatch) and CI resulting from previously generated A. albopictus transinfected lines (<15% hatch) (41, 42). Importantly, the wMelPop infection in HTM can rescue the CI modification (i.e., mod+ resc+) (8), resulting in broods indistinguishable from the HTM × HTM and HTM × HT1 crosses.
Similar to the phenotype in the original D. melanogaster host, the wMelPop infection is associated with reduced adult longevity. HTM longevity assays were conducted at G5 and then repeated at G13. The latter crosses were conducted because prior studies showed an attenuation of the wMelPop phenotype with time (25). The later assay is expected also to ameliorate potential inbreeding effects resulting from transinfection and isoline selection methods. In both the G5 and the G13 assays, HTM female adults were observed to be significantly shorter lived. However, the reduction of female longevity was not as severe as that observed in D. melanogaster, D. simulans, or A. aegypti (25-27), where the life span was approximately halved by wMelPop infection. No effect of wMelPop on adult male longevity was observed.
High maternal transmission of wMelPop is observed in HTM, when outcrossed to uninfected males. However, lower infection frequencies were observed resulting from crosses of HTM females and HTM males. This apparent paternal effect on the Wolbachia infection frequency among progeny is unexpected, and additional study is required to determine whether this represents a direct effect on embryonic infection frequency and/or infection level among progeny or whether this results from an indirect downstream effect (e.g., differential larval competition/survivorship favoring rare uninfected individuals). The absence of paternal transmission was confirmed, which is consistent with prior reports describing that paternal transmission of Wolbachia is rare or absent (19, 24).
Although prior works suggest that, with time, the HTM strain may evolve toward a mutualistic association (25, 26), the current symbiosis is maladaptive. With weak CI and high fitness cost, the wMelPop infection would be unlikely to spread into an uninfected A. albopictus population. The opportunity for expansion is further reduced by the low maternal transmission that results in crosses between HTM individuals. The results, especially in comparison with related work (26), demonstrate the unpredictability of phenotypes resulting in artificial Wolbachia-host associations, which is an important consideration in extending an age structure modification strategy to additional systems (3, 38).
Acknowledgments
We thank S. O'Neill and C. McMeniman for providing the w1118 D. melanogaster strain, which was used as donor material for transinfections.
This research was supported by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print on 9 October 2009.
REFERENCES
- 1.Bonilauri, P., R. Bellini, M. Calzolari, R. Angeflni, L. Venturi, F. Fallacara, P. Cordioli, P. Angelini, C. Venturolli, G. Merialdi, and M. Dottori. 2008. Chikungunya virus in Aedes albopictus, Italy. Emerg. Infect. Dis. 14:852-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchon, D., T. Rigaud, and P. Juchault. 1998. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc. Biol. Sci. 265:1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourtzis, K. 2008. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 627:104-113. [DOI] [PubMed] [Google Scholar]
- 4.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 5.Brelsfoard, C. L., Y. Sechan, and S. L. Dobson. 2008. Interspecific hybridization yields strategy for south pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownstin, J. S., E. Hett, and S. L. O'Neill. 2003. The potential of virulent Wolbachia to modulate disease transmission by insects. J. Invertebr. Pathol. 84:24-29. [DOI] [PubMed] [Google Scholar]
- 7.Callaini, G., R. Dallai, and M. G. Riparbelli. 1997. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J. Cell Sci. 110:271-280. [DOI] [PubMed] [Google Scholar]
- 8.Charlat, S., C. Calmet, and H. Mercot. 2001. On the mod resc model and the evolution of Wolbachia compatibility types. Genetics 159:1415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, P. E., C. J. McMeniman, and S. L. O'Neill. 2008. Modifying insect population age structure to control vector-borne disease. Adv. Exp. Med. Biol. 627:126-140. [DOI] [PubMed] [Google Scholar]
- 10.Dobson, S. L., E. J. Marsland, and W. Rattanadechakul. 2001. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 38:382-387. [DOI] [PubMed] [Google Scholar]
- 11.Dobson, S. L., and W. Rattanadechakul. 2001. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 38:844-849. [DOI] [PubMed] [Google Scholar]
- 12.Dobson, S. L., W. Rattanadechakul, and E. J. Marsland. 2004. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity 93:135-142. [DOI] [PubMed] [Google Scholar]
- 13.Duron, O., and M. Weill. 2006. Wolbachia infection influences the development of Culex pipiens embryo in incompatible crosses. Heredity 96:493-500. [DOI] [PubMed] [Google Scholar]
- 14.Enserink, M. 2006. Infectious diseases: massive outbreak draws fresh attention to little-known virus. Science 311:1085-1086. [DOI] [PubMed] [Google Scholar]
- 15.Gratz, N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18:215-227. [DOI] [PubMed] [Google Scholar]
- 16.Herre, E. A., N. Knowlton, U. G. Mueller, and S. A. Rehner. 1999. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 14:49-53. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, A. A., M. Hercus, and H. Dagher. 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann, A. A., and M. Turelli. 1997. Cytoplasmic incompatibility in insects, p. 42-80. In S. L. O'Neill, A. A. Hoffmann, and J. H. Werren (ed.), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
- 19.Hoffmann, A. A., and M. Turelli. 1988. Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation, and fitness effects. Genetics 119:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam, M. S., and S. L. Dobson. 2006. Wolbachia effects on Aedes albopictus (Diptera: Culicidae) immature survivorship and development. J. Med. Entomol. 43:689-695. [DOI] [PubMed] [Google Scholar]
- 21.Josseran, L., C. Paquet, A. Zehgnoun, N. Caillere, A. Le Tertre, J. L. Solet, and M. Ledrans. 2006. Chikungunya disease outbreak, Reunion Island. Emerg. Infect. Dis. 12:1994-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juchault, P., J. J. Legrand, and G. Martin. 1974. Action inter-spécifique du facteur féminisant responsable de la thélygénie et de l'intersexualité du Crustacé Armadillidium vulgare (Isopode Oniscoide). Ann. Embryol. Morph. 7:265-276. [Google Scholar]
- 23.Kittayapong, P., P. Mongkalangoon, V. Baimai, and S. L. O'Neill. 2002. Host age effect and expression of cytoplasmic incompatibility in field populations of Wolbachia-superinfected Aedes albopictus. Heredity 88:270-274. [DOI] [PubMed] [Google Scholar]
- 24.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2001. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. Biol. Sci. 268:2565-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMeniman, C. J., R. V. Lane, B. N. Cass, A. W. C. Fong, M. Sidhu, Y. F. Wang, and S. L. O'Neill. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141-144. [DOI] [PubMed] [Google Scholar]
- 27.Min, K. T., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neill, S. L., R. Giordano, A. M. E. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16s ribosomal-RNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
- 30.Rai, K. S. 1991. Aedes albopictus in the Americas. Annu. Rev. Entomol. 36:459-484. [DOI] [PubMed] [Google Scholar]
- 31.Reiter, P., D. Fontenille, and C. Paupy. 2006. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect. Dis. 6:463-464. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, K. T., L. J. Thomson, and A. A. Hoffmann. 2003. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics 164:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riegler, M., M. Sidhu, W. J. Miller, and S. L. O'Neill. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15:1428-1433. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, D. B. 1998. Drosophila: a practical approach, p. 1-54. In D. B. Roberts (ed.), Practical approach series 191, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 35.Sasaki, T., T. Kubo, and H. Ishikawa. 2002. Interspecific transfer of Wolbachia between two lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics 162:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon, F., H. Savini, and P. Parola. 2008. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med. Clin. North. Am. 92:1323-1343. [DOI] [PubMed] [Google Scholar]
- 37.Sinkins, S. P., H. R. Braig, and S. L. O'Neill. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. Biol. Sci. 261:325-330. [DOI] [PubMed] [Google Scholar]
- 38.Sinkins, S. P., and F. Gould. 2006. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7:427-435. [DOI] [PubMed] [Google Scholar]
- 39.Tram, U., K. Fredrick, J. H. Werren, and W. Sullivan. 2006. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. J. Cell Sci. 119:3655-3663. [DOI] [PubMed] [Google Scholar]
- 40.Weeks, A. R., M. Turelli, W. R. Harcombe, K. T. Reynolds, and A. A. Hoffmann. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xi, Z. Y., J. L. Dean, C. Khoo, and S. L. Dobson. 2005. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 35:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi, Z. Y., C. C. H. Khoo, and S. L. Dobson. 2006. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. Biol. Sci. 273:1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabalou, S., A. Apostolaki, S. Pattas, Z. Veneti, C. Paraskevopoulos, I. Livadaras, G. Markakis, T. Brissac, H. Mercot, and K. Bourtzis. 2008. Multiple rescue factors within a Wolbachia strain. Genetics 178:2145-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, W. G., F. Rousset, and S. O'Neill. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]