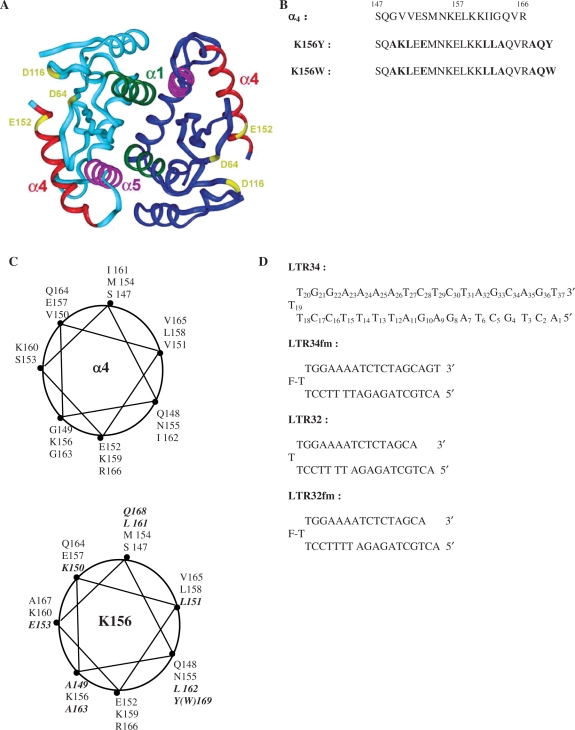
Figure 1.
(A) Crystal structure of the dimeric catalytic core domain showing the α4 helix at the protein surface [after Dyda et al. (10)]. (B) Peptides used in this study: sequences of α4 and analogues K156Y and K156W with the substituted and added residues in bold. (C) Wheel representations of α4 (top) and K156 (bottom) with the substituted and added residues in bold. (D) Oligonucleotides used in this study: unprocessed LTR34 under the hairpin form (three thymine loop and 17 base pair stem) numbered from 5′ to 3′, the processed oligonucleotide LTR32, and their fluoresceinated versions LTR34fm and LTR32fm.