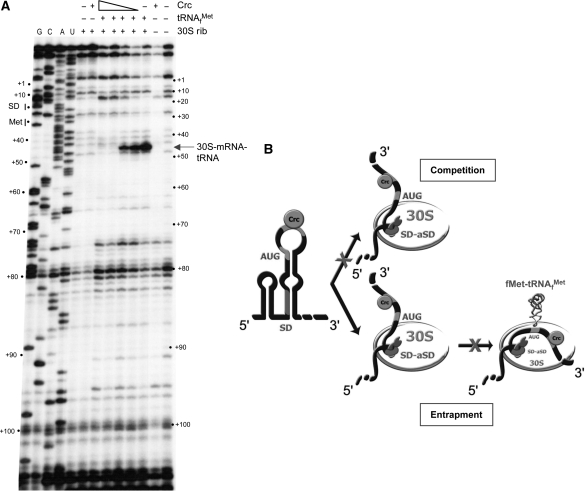
Figure 7.
Crc binding to alkS mRNA prevents the formation of the ternary ribosomal initiation complex. (A) Toeprinting analysis showing the effect of Crc on the formation of the ternary complex formed between alkS mRNA, E. coli ribosomal 30S subunit and the initiator tRNAfMet. The unlabelled alkS mRNA (−37 to +200) was incubated in the absence (−) or in the presence of increasing concentrations of Crc (16 nM, 80 nM, 400 nM or 2 µM), tRNAfMet, or 30S ribosomes, as indicated. The primer extension products were resolved on a 12% urea–polyacrylamide gel, side by side with sequence ladders (lanes G, C, A, U). The toeprint corresponds to tRNAfMet-mRNA-30S complex that stops the elongation of reverse transcriptase (16 nts downstream the A of the AUG initiation codon). Positions relative to PalkS2 transcription start sites are indicated on the sides of the gel. (B) Possible models of the effect of Crc on the formation of the ribosomal initiation complex. The formation of the 30S initiation complex involves as the first step the docking of mRNA structure (formation of the SD helix), followed by the accommodation step that correctly positions the rest of the mRNA to promote stable codon–anticodon interactions in the P-site. Crc binds to the AU rich sequence downstream the AUG codon of alkS mRNA and prevents formation of the ternary complex by blocking the accommodation process. Two mechanisms are possible: the 30S subunit cannot access the mRNA bound to Crc (competition mechanism), or Crc blocks the 30S subunit into an inactive complex (entrapment complex) and prevents the accommodation step to promote the codon-anticodon interaction at the P site. The model of the mRNA binding to the 30S subunit is taken from ref. (37).