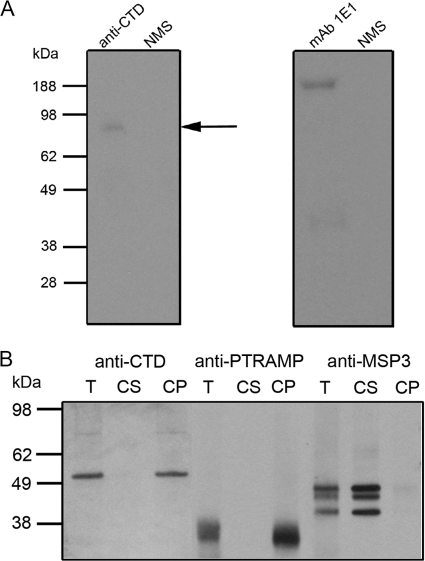
FIG. 3.
Membrane anchorage of GAMA. (A) To provide evidence of a GPI anchor, antibodies were used to immunoprecipitate full-length GAMA (∼83 kDa, arrow) from [3H]glucosamine- and mannose-labeled parasites. As a positive control, the MAb 1E1 was used to immunoprecipitate full-length MSP1 (∼200 kDa) from labeled parasites. The proteins immunoprecipitated with these two antibodies were fractionated on different gels, and the film was subjected to different exposure times. No proteins were immunoprecipitated with normal mouse serum (NMS). (B) To examine whether or not GAMA is membrane bound, total schizont material (T) was treated with a high-pH carbonate buffer to yield carbonate-soluble (CS) and -insoluble (CP) fractions, which were then resolved by SDS-PAGE and analyzed by Western blotting. Anti-CTD antiserum detected two species, both of which were found in the carbonate-insoluble fraction, confirming the membrane association. The larger species is presumably full-length GAMA, and the 49-kDa polypeptide (p49) likely represents the product of a proteolytic cleavage event. Blots were also probed with an antibody raised against the type I transmembrane protein PTRAMP and the soluble MSP3 in order to verify the outcome of the extraction procedure.