TABLE 4.
Cyclization Cascade of Substituted Vinyl (o-Azidophenyl)allenes To Furnish Indole and Pyrrole Products
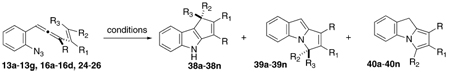 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 110 °Ca |
hvb |
hv/CuIc |
|||||||||||
| entry | allenyl azide | R | R1 | R2 | R3 | 38d(%) | 39d(%) | 40d(%) | 38d(%) | 39d(%) | 40d(%) | 38d(%) | 39d(%) |
| a | 13a | CH3 | H | H | H | 40 | 56 | 24 | 20 | 42 | |||
| b | 13c | (CH2)2OTBS | H | H | H | 52 | 43 | 24 | 16f | 36 | |||
| c | 13d | t-Bu | H | H | H | 57 | 20 | 53 | 38 | 59 | |||
| d | 13b | CH2OTBS | H | H | H | 22 | 29 | 24 | 33 | 55 | 3 | ||
| e | 13e | CH3 | Ph | H | H | 40 | 30 | 44 | 31 | 67 | |||
| f | 13f | CH3 | CH3 | H | H | 36 | 36 | 38 | 35 | 54 | |||
| g | 13g | Ph | CH3 | H | H | 47 | 39 | 23 | 11e,f | 69f | |||
| h | 23 | CH3 | H | CH2OTBS | H | 24 | 34 | decomp | decomp | ||||
| i | 25 | CH3 | H | TBS | H | 33 | 7 | 25 | 9 | 44f | |||
| j | 16a | CH3 | H | Ph | H | (33)e | 35 | decomp | decomp | ||||
| k | 16c | CH3 | –(CH2)3– | H | (36)e | 40 | not examined | not examined | |||||
| l | 16d | CH3 | –(CH2)4– | H | 36 | 51 | 32 | 27 | 54 | ||||
| m | 24 | CH3 | CH3 | (CH2)2OTBS | H | 7 | 40 | (20)e,g | (40)e,g | 42g | (42)e,g | ||
| n | 16b | CH3 | Ph | Ph | Ph | 45 | 21 | 51 | 31 | 53 | 26 | ||
100 mM in toluene.
254 nm in CH3CN (5 mM).
254 nm in CH3CN (5 mM), 150 mol % of CuI.
Isolated, chromatographically purified material.
Not isolated; estimated by integration of the 1H NMR spectrum of the crude reaction mixture.
Irradiated at 300 nm.
Irradiated at 350 nm.
