TABLE 5.
Discovery and Optimization of the Cui-Mediated Photochemical Cascade Cyclization of 29a into 42b
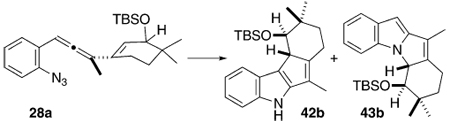 | |||||
|---|---|---|---|---|---|
| entry | irrada(nm) | heatb(°C) | conditions | yieldc(%) | 42b/43b ratiod |
| a | 110 | no additives | 80 | 1:1.6 | |
| b | 254 | no additives | 67 | 1:1 | |
| b | 254 | excess CuI, LiBr, Mg powder | 33 | >10:1 | |
| d | 254 | LiBr (1 equiv) | 68 | 1:1.1 | |
| e | 254 | Mg powder | (33)e | 1:1.2 | |
| f | 254 | LiBr (1 equiv), CuI (0.05 equiv) | f | 1:1.4 | |
| g | 254 | LiBr (1 equiv), CuI (1 equiv) | 21 | 5.4:1 | |
| h | 254 | CuBr·SMe2 (1 equiv) | f | 2.8:1 | |
| i | 254 | CuBr·SMe2 (0.25 equiv) | (17)e | 1.6:1 | |
| j | 254 | Cu(OAc)2 (1 equiv) | f | 5:1 | |
| k | 254 | CuI (0.5 equiv) | 67 | 4:1 | |
| l | 254 | CuI (0.8 equiv) | 72 | 6: 1 | |
| m | 254 | CuI (1.2 equiv) | 67 | 9:1 | |
| n | 254 | CuI (1.5 equiv) | 70 | 10:1 | |
| o | 110 | CuI (1.5 equiv), 5 mM | 75 | 1:1.6 | |
| p | 110 | CuI (1.5 equiv), 35 mM | 66 | 3.5:1 | |
| q | 110 | CuI (1.5 equiv), 51 mM | 65 | 5:1 | |
| r | 254 | [Rh(OAc)2]2 (1 equiv) | (48)e | 1:1 | |
Irradiated through a quartz vessel as a 5 mM CH3CN solution of 28a.
100 mM solution in toluene, unless otherwise noted.
Isolated, chromatographically purified material. The N-cyclized material had undergone elimination to furnish the diene 44b (see Table 6).
Ratio by integration of the 1H NMR spectrum of the crude reaction mixture prior to chromatography.
Only the N-cyclized material was isolated; 42b decomposed upon attempted chromatography.
Products were not isolated.
