Figure 1.
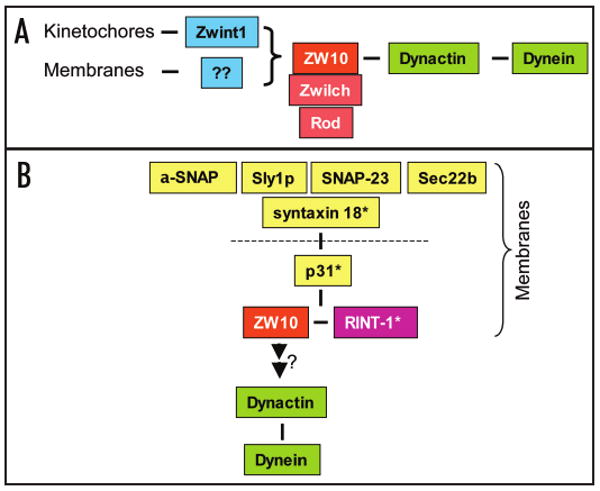
Diagrammatic representation of ZW10-containing complexes. (A) ZW10 exists as a coherent 20S complex, which contains Zwilch and Rod as components. ZW10 itself interacts with the dynamitin subunit of the dynactin complex, which in turn interacts through its p150Glued subunit with the base of the dynein molecule. ZW10 is shown anchored to the kinetochore through Zwint-1. Recent evidence indicates that ZW10 also participates in recruiting dynactin and dynein to membranous and cell cortical structures, though how ZW10 is anchored in these cases is unknown. (Other dynein/dynactin interactors involved in its association with membranes are not been indicated in this scheme for simplicity.) (B) An apparently independent complex has been identified associated with the t-SNARE syntaxin-18. This complex can be dissociated (dotted line) by NSF and α-SNAP to yield α subcomplex containing p31, ZW10 and RINT-1. Whether ZW10 participates in SNARE function, links dynein to membranes, or links SNAREs to dynein remains to be explored more fully. Although a ZW10 homologue has not been identified in yeast, potential orthologues of syntaxin-18 and others of its interactors are known in this organism (noted by *): syntaxin-18 = Ufe1p; p31 = Use1p; and RINT-1 = Tip20p (See text for details).
