1. Introduction
As a population, long-term care (LTC) residents have many limitations that may contribute to weight loss and nutritional problems including decreased functional status and dysphagia; others have already linked poor nutritional status to some psychiatric disorders including depression and dementia (Buchanan, Wang, Tai-Seale, & Ju, 2003; Chouinard, Lavigne, & Villeneuve, 1998). Reduced food intake in older adults can be clinically related to poor appetite, chronic illness, dementia (Chouinard et al., 1998; Gil Gregorio, 2003), neurosensory loss, and poor oral/dental health (Baumgartner, 2000; Guigoz, Lauque, & Vellas, 2002; Nordenram & Ljunggren, 2002). Iatrogenic reasons for malnutrition in long-term care facilities include too few nursing assistants to assist at mealtime (Kayser Jones & Schell, 1997), poor quality food (Crogan, Evans, Severtsen, & Shultz, 2004), and polypharmacy (Evans, Crogan, & Shultz, 2004; Fabiny & Kiel, 1997; Perry & Turner, 2001). All these factors contribute to malnutrition being a significant clinical problem in long-term care with estimates ranging from 30 to 85% of elders (Crogan & Pasvogel, 2003). The primary objective of this study was to evaluate the association between delirium and under-nutrition in nursing home elders.
1.1 Theoretical Framework
Delirium is characterized by an acute onset of symptoms that include disturbances in orientation, levels of consciousness, memory, attention, thought, behavior, and the sleep–wake cycle, with specific physiologic factors underlying the problem (Inouye, Rushing, Foreman, Palmer, & Pompei, 1998). In general, these clusters of symptoms follow a fluctuating course and can easily be mistaken for dementia by the novice clinician. The scope of the delirium problem in LTC is exacerbated by a higher prevalence of known risk factors for delirium, notably dementia, severity of illness, comorbidity, and polypharmacy (Cacchione, Culp, Dyck, & Laing, 2003; McCusker, Cole, Dendukuri, Han, & Belzile, 2003). Recent work has suggested under-nutrition, specifically low body mass index (BMI) and protein malnutrition negatively influence quality of life and other outcomes. (Crogan & Pasvogel, 2003).
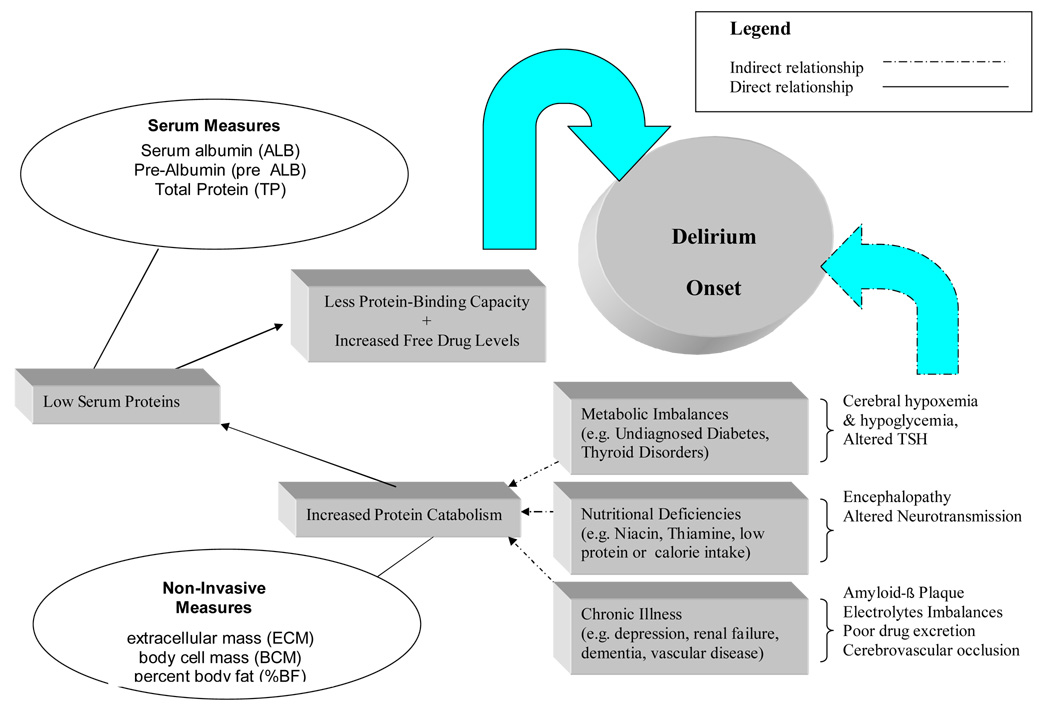
Figure 1 conceptually displays the role of poor nutrition and delirium onset. First of all, it is biologically plausible that many disease states lead to low serum proteins or interfere with cognitive functioning via altered oxygen, glucose, and low perfusion pressure to the cerebrum (Foreman, Wakefield, Culp, & Milisen, K. (2001). These conditions include metabolic disorders, low calorie consumption, nutrition, cerebrovascular disease and chronic illnesses such as hypertension and renal disease. In long-term care, there are other problems such as depression, dementia, stroke, and poor swallowing that also impact on nutritional intake (Crogan & Corbett, 2002). Protein catabolism alters several nutritional assessment parameters including anthropometric measures and serum chemistry, but there are no on-site laboratories to measure serum albumin in many nursing homes; they simply merely rely on a weight without a body composition calculation (Fabiny & Kiel, 1997; Friedmann, Elasy, & Jensen, 2001; Guigoz et al., 2002).
Figure 1.
Dual Negative Influence of Malnutrition on Delirium Onset in Frail Nursing Home Elders
A major problem with protein catabolism and the resulting lowered plasma protein is a rising serum drug level in medications that are normally protein bound (van Vliet, Schuurmans, Grypdonck, & Duijnstee, 2006). Medication overdose and polypharmacy are frequent problems in nursing homes; some common medications have anticholinergic properties which are known to contribute to delirium (Holden & Kelly, 2002; McCusker, Cole, Dendukuri, Han, & Belzile, 2003).
Micronutrients deficiencies in mineral and trace elements, antioxidants, and other vitamin deficiencies such as B12 status (Ledikwe, Smiciklas-Wright, Mitchell, Miller, & Jensen, 2004; Ortega et al., 1997; Wendland, Greenwood, Weinberg, & Young, 2003) are also known to impact on delirium onset. However, it is predominately the low serum protein and polypharmacy (i.e. excessive medication) that are the most dangerous coupling of factors in delirium. Under-nutrition clearly impacts on the quality of care in the nursing home (Crogan & Pasvogel, 2003). Since many long-term care facilities do not have medical laboratories to directly measure serum protein, it appeared practical to include some non-invasive measures of nutrition in our protocols to assess nutritional status as these might be more readily implemented in long-term care.
1.2 Nutrition Assessment
Of those long-term care studies dealing with nutrition and weight loss in the United States, most use the Minimum Data Set (MDS) and not direct clinical observations of weight or cognitive status (Corbett, Crogan, & Short, 2002; Crogan & Corbett, 2002; Keller & Hirdes, 2000). The MDS is a federally mandated comprehensive assessment form completed on each nursing home resident in the United States. Nursing staffs in LTC are challenged with multiple tasks and limited personnel and don’t take the time to complete the assessment (Davidson & Getz, 2004). Currently, federal regulators in the U.S. require little more than documentation regarding weight loss (Corbett et al., 2002). Studies of body composition in younger and middle-aged adults in the United States are usually based on anthropometric data with measures of body weight, stature, skinfold thicknesses, and circumferences (Maitland, Myers, Hipp, Hayes, & Greenspan, 1993; Shaikh & Mahalanabis, 2004; Tai, Ho, Fok, & Tan, 1999). These measures provide correlates of fatness (i.e. body mass index; BMI) or estimates of total body fat (Chumlea et al., 2002), but older adults are rarely included in these samples. Some of these methods have been challenged as to their accuracy when compared to more advanced techniques (Piers, Soares, Frandsen, & O'Dea, 2000).
Bioelectrical impedance analysis (BIA) can be used to estimate body composition, although it has been neglected as a technique in this setting (Culp, Mentes, & Wakefield, 2003). Simply described, BIA is a technique whereby a very small electrical signal carried by water and fluids is passed through the body; impedance is greatest in fat tissue, which contains only 10–20% water (Di Iorio, Scalfi, Terracciano, & Bellizzi, 2004). Fat-free body mass, which contains 70–75% water, allows the signal to pass much more easily.
1.3 Hypotheses
The significance of any association between declining nutritional status and delirium in older nursing home adults is poorly articulated in the literature, despite widespread interest on addressing dietary intake in long-term care elders. Some have linked poor nutrition to mortality (Hirdes, Frijters, & Teare, 2003; Volpato et al., 2004) or focused on tube feedings and end stage dementia in nursing home patients (Gessert & Calkins, 2001; Mitchell, Kiely, & Gillick, 2003; Murray, 2000; Okada et al., 2001; Sheiman & Pomerantz, 1998; Silver, Wellman, Arnold, Livingstone, & Byers, 2004). The following hypotheses are proposed:
Participants who screen positive for delirium will have less body fat (%BF, % FFM, %ECM, and %BCM) than those without delirium.
Participants who screen positive for delirium will have lower serum albumin and total protein levels than those without delirium.
Participants with dementia (mean Mini-Mental Status score < 19) will have less body fat (%BF, % FFM, %ECM, and %BCM) than those without dementia.
2. Methods
2.1 Design
A longitudinal design with a 28-day period of follow-up was used because of the fluctuating course of delirium. In order to reduce systematic error introduced by only using one nursing care routine and one primary care model, we elected to complete a multi-center study over a 3-year period. We limited the duration to 28 days because of the high participant response burden. Other approaches to reducing burden placed on our participants was to divide the 28 days into two phases; Phase I consisted of a 14-day intensive delirium surveillance with clinical screenings completed three times per day and Phase 2 where screening decreased to once a day. All delirium screenings, BIA measurements, and weights were completed by our own registered nurse research assistants (i.e. not members of facility staff). All serum protein studies were completed by our own lab vendor so that there would be no variability between albumin testing equipment.
2.2 Sample
A two-stage cluster sampling approach was used in a defined southeast Iowa catchment area. The first stage was to randomly select six counties from a region of fifteen counties. All counties and all facilities had an equal opportunity to participate. The total sampling frame consisted of forty-five LTC facilities with 3,554 operating beds. The second “cluster” was a sampling procedure based on facility size; half of the participating facilities contained 75 beds or fewer and half had more than 75 beds.
2.2.1 Eligibility Criteria
All participants were in a skilled or intermediate care bed for at least 30 days, aged 65 years or older, and were required to read, write, and speak English. Severely demented patients who could not accomplish the latter were excluded. Residents with head trauma, brain tumor, toxin-related neurological disorder, and an implanted defibrillator also were excluded. All protocols were reviewed by the university Institutional Review Board (IRB), and consent was obtained from either the participant or his or her legal guardian as appropriate. A sample size of 312 was obtained from 13 facilities, however one person was dropped from the analysis due to a failure to complete all of the nutrition indices studied, thus the final sample size was n = 312. The mean age for the total sample was 86.1 years (SD = 7.17). Men represented 23.6% (n = 73) of the sample and women represented 76.4% (n = 239).
2.3 Screening Instruments
Attention was measured with the Vigilance A that consists of a series of 60 letters read to the participant (Strub & Black, 1985). When the letter “A” is spoken by the research nurse, the subject gestures he or she has heard it. Two or more errors are considered abnormal In the event the dementia participants had a baseline of 2 errors; we altered the scoring so that their errors exceeded their baseline.
In order to detect chronicity in mental status from baseline and to identify chronically confused subjects, we used the Mini-Mental State Exam (MMSE) which has been used widely in neurological research protocols (Irons, Farace, Brady, & Huff, 2002; Jagmin, 1998; O'Neill, 1991; Solomon et al., 1999). A MMSE score of 19 is generally considered to be associated with some form of dementia or cognitive impairment if the result is consistent over several measurement occasions (Lamarre & Patten, 1994).
The NEECHAM confusion scale (Neelon, Champagne, Carlson, & Funk, 1996), was used to screen for delirium, but interpreted in congruence with other measures (see below). The NEECHAM contains many of the DSM (Diagnostic and Statistical Manual of Mental Disorders) criteria for delirium (Csokasy, 1999; Johansson, Hamrin, & Larsson, 2002; Matsushita, Matsushima, & Maruyama, 2004; Neelon et al., 1996); thus establishing external validity. High internal consistency (0.90) and inter-rater reliability (0.96 to 0.99) have been established in both acute and long-term care (Cacchione, 2002; Neelon, et al., 1996). The NEECHAM total score ranges from 0–30; a score of less than 24 constitutes a positive delirium screen.
We used a second screening instrument, the Confusion Assessment Method (CAM) which operationalizes the clinical features of the delirium diagnosis as identified in the DSM (Inouye et al., 1990). The CAM has a dichotomous positive or negative result; external validity through expert review in both geriatric psychiatry (Jackson & Ely 2003) and gerontological nursing (Laplante & Cole, 2001). The screen positive for the CAM requires the presence of features A and B and either C or D (listed below):
Acute onset of confusion and fluctuating course
Inattention
Disorganized Thinking
Altered Level of Awareness
2.3.1 Positive Delirium Screen Criteria
It is extremely difficult to clinically delineate between delirium and dementia in long-term care. While there is no one way to do this, we emphasize that we are screening only and that no expert clinical diagnosis of delirium was made in this study. For operational purposes, in order for a participant to be identified as screening positive for delirium in this study the following criteria were required:
Vigilance A error score > baseline as described above.
Decline of two or more points on the Mini-Mental Status from baseline.
NEECHAM score of 24 or less.
Confusion Assessment Method (CAM) screen positive
2.3.2 Other Measures
The Geriatric Depression Scale-short form (GDS), a 15 item yes-no format questionnaire was used to evaluate affect; the cut –off score of ≥ 5 yielded the best sensitivity and specificity; 0.93 and 0.48 respectively (Burke, Roccaforte, & Wengel, 1991; Lesher & Berry hill, 1994; Sheikh et al., 1991). Cut-off points for the GDS were scores of 0–4 as non-depressed, score of 5–9 was mild depression, and a score of 10–15 represented moderate to severe depression (Lesher & Berry hill, 1994).
Nutritional variables were measured using BIA, height, and weight (WT) to calculate body mass index (BMI). WT and BIA were taken at baseline and follow-up days 7, 14, and 28 for body weight (WT). Estimates from the BIA procedure included body cell mass (BCM), extracellular mass (ECM), percent body fat (BF) and fat free mass (FFM). FFM is the summed BCM and ECM. BCM is the total mass of all the cellular elements in the body, and therefore, represents the metabolically active component of the body, including oxygen consumption, carbon dioxide production, glucose oxidation, and protein synthesis (Volpato et al., 2004). ECM is the support mass of the body and is metabolically inactive. ECM is consisted of extracellular fluids and solids, such as bone and cartilage; the primary function is one of support and transport. BIA measurements were taken at least 2 hours after meals and at least 6 hours after diuretic medication.
2.3.3 Serum Protein Levels
Serum albumin and total protein blood levels were assessed at baseline and day 14. These specimens were analyzed at a central laboratory with a Roche Cobas Integra 700© Clinical Chemistry Analyzer. The unit of measure was grams per deciliter (g/dL); serum albumin was measured by the bromcresol green (BCG) method to ensure consistency in interpretative values (Morris, MacIntyre, & Redington 1998). Serum prealbumin was measured on baseline and day 14 only due to the half-life of this physiological parameter. All serum specimens were transported on ice to the laboratory and were analyzed within 4 hours of venipuncture.
2.4 Data Analysis
A one-way analysis of variance (ANOVA) was used to detect differences for the NEECHAM and MMSE scores by grouping participants by nutritional indices. Repeat measure ANOVA was used as dependent variables were measured on baseline, day 7 and day 14. An estimated the odds ratio with 95 percent confidence intervals was used for detecting delirium. Cut points for BMI were set at <22, 22–28, and >28 and are generally considered to classify lean, normal, and obese results (Wilson, D'Agostino, Sullivan, Parise, & Kannel, 2002 ). The raw measures for %BF, %FFM, %ECM, and %BCM were derived by dividing the individual’s body weight into each of these parameters so that a percent is derived for comparison purposes.
3. Findings
The %FFM by gender and BMI group is displayed in Table 1. One would expect that participants with a low BMI (e.g. < 22) would have a higher FFM value than those with normal BMI (22–28) and higher BMI (> 28) and this was the case in this sample. We conducted a repeat measure ANOVA for each of the four BIA observation time points (baseline, day 7, day 14, and day 28) for BCM, ECM, and FFM. There were no significant differences between time points for BCM, ECM, BF, and FFM when gender was entered into the model.
Table 1.
Body Weight Percent Fat Free Mass (FFM) Calculated by BIA Compared with Traditional BMI Classification
| BMI | N | Mean % FFM | SD | F | p |
|---|---|---|---|---|---|
| Women | |||||
| < 22 | 58 | 79.29 | 7.36 | 92.0 | <.001 |
| 22–28 | 92 | 69.57 | 8.49 | ||
| > 28 | 89 | 59.58 | 9.65 | ||
| Total | 239 | 68.21 | 11.55 | ||
| Men | |||||
| < 22 | 10 | 81.91 | 6.15 | 21.5 | <.001 |
| 22–28 | 23 | 67.69 | 9.51 | ||
| > 28 | 40 | 59.31 | 11.00 | ||
| Total | 73 | 65.04 | 12.5 | ||
Note:: BIA = Bioelectrical Impedance Analysis; FFM = Fat Free Mass; BMI = Body Mass Index, weight in kg divided by height in meters squared
3.1 Delirium & Nutritional Status
There were 69 from a total of 312 participants who screened positive for delirium (21.8%) during the 28 days of surveillance. The mean age for residents with delirium was 88.5 years (SD = 6.13) compared to 85.5 years for those without delirium (SD = 7.25), which was significant (t = −3.03, p <.001). Delirium was slightly more common among men (n = 19, 26.0%) than women (n=49, 20.5%). Mean length of stay in the LTC facility was shorter for delirium cases (769.5 days, SD = 665.7) than for non-cases (856.2 days, SD = 946.3), but was not significant. The predefined cut-points for BMI revealed statistically significant differences in the mean NEECHAM scores for each subject across the entire surveillance period. Participants with a BMI below 22 had lower mean NEECHAM scores (M = 25.72, SD = 3.36, n = 68, p < .05) than those with a 22–28 BMI (M = 26.72, SD = 2.93, n = 115) or a BMI > 28 (M = 26.58, SD = 2.34, n = 129). The odds ratios for delirium is presented in Table 2, with significantly increased odds of screening delirium positive for %FFM, %ECM, and %BCM when stratified by gender. These odds ratio greater > 1.0 would indicate that as an elderly nursing home participant became more “lean” the risk of delirium was increased, however the finding of increased risk with %BCM is noteworthy from a delirium pathogenesis perspective (see discussion).
Table 2.
Odds Ratio for Delirium and Body Composition Measure by Gender
| % BF | % FFM | % ECM | % BCM | |
|---|---|---|---|---|
| Women | ||||
| Odds Ratio | 0.99 | 1.024* | 1.033* | 1.050* |
| 95 % CI | .97–1.02 | 1.015–1.020 | 1.025–1.041 | 1.038–1.062 |
| Men | ||||
| Odds Ratio | 0.97 | 1.016* | 1.029* | 1.042* |
| 95 % CI | .93–1.01 | 1.009–1.020 | 1.015–1.043 | 1.021–1.063 |
p < .001
Note: % BF = percent of participant’s weight for body fat
% FFM = percent of participant’s weight for fat free mass
% ECM = percent of participant’s weight for extra-cellular mass
% BCM = percent of participant’s weight for body cell mass
3.2 Dementia & Nutritional Status
Participants were stratified by mean MMSE score into 3 classes across the 28-day period. Persons considered to have moderate to severe dementia were those with a mean score of < 19 on their MMSE compared to those with mild dementia (MMSE M score =19–23) and no evidence of dementia (MMSE M score > 23). Those in the lowest MMSE score group were more likely to measure the lowest %FM (F = 4.70, p = 0.01) and the highest %FFM (F = 4.68, p = 0.01) as displayed in Table 3.
Table 3.
Mini-Mental Status by Percent Body Weight Body Composition
| N | Mean | SD | F | P | |
|---|---|---|---|---|---|
| % FM | |||||
| MMSE <19 | 83 | 29.25 | 11.75 | 4.70 | 0.01 |
| MMSE 19–23 | 68 | 32.88 | 12.32 | ||
| MMSE >23 | 161 | 34.09 | 11.42 | ||
| % FFM | |||||
| MMSE <19 | 83 | 70.75 | 11.75 | 4.68 | 0.01 |
| MMSE 19–23 | 68 | 67.12 | 12.33 | ||
| MMSE >23 | 161 | 65.92 | 11.43 | ||
Note: FM = Fat Mass;
FFM = Fat Free Mass;
MMSE = Mini-Mental Status Exam
3.3 Serum Protein & Delirium
Serum albumin levels and delirium results are displayed in Table 4 for baseline; day 7 and day 14 of follow-up where repeat measure ANOVA results were significant. Delirium positive participants had lower albumin levels than the non-delirium group (F = 3.55, p = .06). Repeat measure analysis for serum pre-albumin and total protein was not statistically significant, so only baseline was used. Positive screen delirium participants also had lower pre-albumin levels at baseline (M = 22.91 mg/dL, SD = 7.38) compared to those without delirium (M = 23.81 mg/dL, SD = 7.40), but pre-albumin did not significantly increase the risk when analyzed as a continuous variable (OR = 1.017, 95% CI 0.98–1.06). Baseline total protein levels were also slightly lower in delirium cases (M = 6.86 g/dL, SD = .57) compared to non-cases (M = 6.88 g/dL, SD = .60), but this also was not statistically significant.
Table 4.
Repeat Measure ANOVA For Serum Albumin by Delirium Status
| Time | Mean (g/dL) | SD | F-Value | P | |
|---|---|---|---|---|---|
| Baseline | Delirium | 3.70 | 0.33 | 3.55 | 0.06 |
| No Delirium | 3.79 | 0.37 | |||
| Day 7 | Delirium | 3.74 | 0.40 | ||
| No Delirium | 3.80 | 0.37 | |||
| Day 14 | Delirium | 3.66 | 0.36 | ||
| No Delirium | 3.78 | 0.39 | |||
3.4 Depression
Depression scores measured with the GDS indicated that 53.8 % (n = 168) were negative, 36.9% (n = 115) demonstrated mild depression, and 9.3% (n = 29) were moderate to severely depressed. There were no differences in GDS scores between those with delirium (M = 5.06, SD = 3.45) and those without delirium (M = 4.52, SD = 3.02). There were no differences in %FFM, %BCM, %ECM, or %BF by GDS classification. We did find significantly lower serum total protein levels in the moderate to severely depressed compared to those who screened negative and those with mild depression (F = 3.75, p = 0.024) as displayed in Table 5. There were no differences in NEECHAM scores by depression outcome rating across the three groups (i.e. negative, mild, and moderate to severe depression).
Table 5.
Serum Albumin, Pre-Albumin, and Serum Protein Levels by Depression Rating
| Serum Measure | GSD Outcome | Mean | SD | 95% Confidence Interval for Mean | F | p | |
|---|---|---|---|---|---|---|---|
| Albumin g/dL | Negative | 3.79 | .357 | 3.74 | 3.85 | 1.01 | NS |
| Mild | 3.77 | .407 | 3.69 | 3.84 | |||
| Mod -Severe | 3.69 | .260 | 3.59 | 3.79 | |||
| Pre-Albumin mg/dL | Negative | 24.31 | 7.85 | 22.93 | 25.69 | 1.99 | NS |
| Mild | 22.91 | 6.90 | 21.54 | 24.28 | |||
| Mod -Severe | 21.61 | 6.83 | 18.96 | 24.26 | |||
| Total Protein g/dL | Negative | 6.85 | .522 | 6.77 | 6.93 | 3.75 | 0.024 |
| Mild | 6.96 | .639 | 6.85 | 7.08 | |||
| Mod -Severe | 6.65 | .521 | 6.46 | 6.85 | |||
g/dL = grams per deciliter
mg/dL = milligrams per deciliter
GDS= Geriatric Depression Scale, scored as follows: < 5 Negative, 5–9 Mild, 10–15 Moderate to Severe
4. Discussion
The 21.8% delirium prevalence found here is of great concern as delirium is “camouflaged” in long-term care among persons with dementia (Pisani, Redlich, McNicoll, Ely, & Inouye, 2003; Rodriguez-Martin, Qizilbash, & Lopez-Arrieta, 2001). When we compared the data from the BIA analysis by age and gender, an increased risk for delirium was identified for both men and women who were leaner or have a lower % FFM; a similar significant finding demonstrated a related decrease in % BCM as increasing delirium risk.
The decrease %BCM in delirium-positive participants is especially alarming (Roubenoff, 1999). A decrease is BCM is found in elders with protein-calorie malnutrition; it is also a characteristic of viral infections and sepsis when there is a greater decrease in the BCM than in the ECM (VanderJagt, Harmatz, Scott-Emuakpo, Vichinsky, & Glew, 2002). The % BCM and % ECM are proxy measures for the body’s pharmacokinetics; specifically the absorption, distribution, metabolism and excretion of medications (Dharmarajan, Tankala, Patel, Sipalay, & Norkus, 2001; Dickson, 1991). BCM could represent the metabolically active component of the brain associated with known risk factors for delirium including poor oxygenation, low glucose levels, and decreased protein synthesis associated with drug toxicities (Foreman, et al., 2001; Levkoff, Cleary, Liptzin, & Evans, 1991).
A geriatric syndrome referred to as “failure to thrive” and is a risk with the trends seen here between body composition and depression (Robertson & Montagnini, 2004). Others have found an 11% depression prevalence using the MDS (Brown, Lapane, & Luisi, 2002)., but 38% of this sample scored in the mild depressive range and over 9 % in the moderate to severe depression range.
4.1.1 Clinical Implications
The implications for nursing practice relate to the need for better nutritional assessment in older adults in long-term care, especially those with delirium. The study revealed some noninvasive techniques for assessing the physiological aspects of nutritional status; however some of the traditional approaches like monitoring weight and other anthropological techniques (e.g. the Quetelet body mass index and the hip to waste ratio) may need to be used depending on the resources of the institution. Preventing under-nutrition in frail elders in long-term care most likely will decrease the incidence of delirium and improve clinical outcomes in other areas (e.g. skin integrity and wound healing). Since some cases of delirium are drug-induced; individuals who are under-nourished are more prone to confusion.
Pharmacodynamics, the study of what a drug does to the human system, is an important consideration in the pathogenesis of delirium. Some medications are transported in a protein-bound state and when albumin or total protein levels are low the individual is at increased risk for delirium. There is a tremendous need for interdisciplinary collaboration between the physician, pharmacist, and nursing to prevent the phenomena of polypharmacy. Often times nursing home clients are prescribed medications and no one reviews the efficacy or need at a future date. For example, dementia patients on acetylcholinesterase inhibitors for several years may be inappropriate when there is little effectiveness for improving memory in many of these agents for this duration of time (Holden & Kelly, 2002). We recommend drug-utilization reviews by an interdisciplinary team at least every 6 months, perhaps even more frequently in nursing home residents with 5% or greater loss in body mass since admission to the long-term care facility. It is also our recommendation that advanced practice registered nurses are best able to conduct bedside assessments for delirium in long-term care and participate in this drug-utilization review process (Jehan & Nelson, 2006).
4.1.2 Study Limitations & Future Directions
The sample contained persons 90 years or older; there are no standardized measures for BIA data from healthy older adults for this age stratification. Clinical research is challenging in this age group, many have diuretic medications and this complicated our body composition measures. Although dementia nursing home residents were included in these protocols, we did not sample severely demented persons who could not read and write as these were skills needed to complete some of our cognitive measures. It was extremely difficult to move our research staff between counties and facilities over the life of this study. Repetition of questions in the frequent screening of delirium could have led to rote memorization of expected responses, thus elders may have been able to conceal memory and other transient cognitive symptoms. The very frequency of our interactions could have provided a stimulus to improve attention.
In terms of future research, we did not note any seasonal variations with the number of delirium-positive screenings. We cannot over-emphasize the compelling need to conduct clinical research and not rely on secondary data sources when studying delirium in long-term care elders (Foreman et al, 2001). Although the Minimum Data Set that is required in the United States for federal payment of long-term care services does contain delirium indicators, we do see competence issues and lack of external validation in identifying delirium symptoms in the nursing personnel at these facilities (Rapp et al, 2001).
4.2 Summary
Long-term care elders often present with under-nutrition or become undernourished during their stay in long-term care. These BIA results demonstrated an increased risk for delirium in elders with increased % of FFM, %ECM deterioration, but to an even greater extent deterioration of %BCM. BCM represents the metabolically active component of the body, including oxygen consumption, glucose oxidation, and protein synthesis; all of these are considered as precursors in the pathogenesis of delirium.
ACKNOWLEDGMENTS
Funded in part from the National Institute on Aging, 1 R01 AG17939-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. American Journal of Epidemiology. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN. Body composition in healthy aging. Annals New York Academy of Science. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- Bourdel-Marchasson I, Vincent S, Germain C, Salles N, Jenn J, Rasoamanarivo E, Emeriau JP, Rainfray M, Richard-Harston S. Delirium symptoms and low dietary intake in older inpatients are independent predictors of institutionalization: a 1-year prospective population-based study. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59(4):350–354. doi: 10.1093/gerona/59.4.m350. [DOI] [PubMed] [Google Scholar]
- Brown MN, Lapane KL, Luisi AF. The management of depression in older nursing home residents. Journal of the American Geriatrics Society. 2002;50(1):69–76. doi: 10.1046/j.1532-5415.2002.50010.x. [DOI] [PubMed] [Google Scholar]
- Buchanan RJ, Wang S, Tai-Seale M, Ju H. Analyses of nursing home residents with multiple sclerosis and depression using the Minimum Data Set. Multiple Sclerosis. 2003;9(2):171–188. doi: 10.1191/1352458503ms872oa. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. J Geriatric Psychiatry Neurology. 1991;4(3):173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- Cacchione PZ, Culp K, Dyck MJ, Laing J. Risk for acute confusion in sensory-impaired, rural, long-term-care elders. Clinical Nursing Research. 2003;12(4):340–355. doi: 10.1177/1054773803253917. [DOI] [PubMed] [Google Scholar]
- Chouinard J, Lavigne E, Villeneuve C. Weight loss, dysphagia, and outcome in advanced dementia. Dysphagia. 1998;13(3):151–155. doi: 10.1007/PL00009565. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. International Journal of Obesity-Related Metabolic Disorders. 2002;26(12):1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- Corbett CF, Crogan NL, Short RA. Using the minimum data set to predict weight loss in nursing home residents. Applied Nursing Research. 2002;15(4):249–253. doi: 10.1053/apnr.2002.35947. [DOI] [PubMed] [Google Scholar]
- Crogan NL, Corbett CF. Predicting malnutrition in nursing home residents using the minimum data set. Geriatric Nursing. 2002;23(4):224–226. doi: 10.1067/mgn.2002.126972. [DOI] [PubMed] [Google Scholar]
- Crogan NL, Corbett CF, Short RA. The minimum data set: Predicting malnutrition in newly admitted nursing home residents. Clinical Nursing Research. 2002;11(3):341–353. doi: 10.1177/105477380201100308. [DOI] [PubMed] [Google Scholar]
- Crogan NL, Evans B, Severtsen B, Shultz JA. Improving nursing home food service: Uncovering the meaning of food through residents' stories. Journal of Gerontological Nursing. 2004;30(2):29–36. doi: 10.3928/0098-9134-20040201-07. [DOI] [PubMed] [Google Scholar]
- Crogan NL, Pasvogel A. The influence of protein-calorie malnutrition on quality of life in nursing homes. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(2):159–164. doi: 10.1093/gerona/58.2.m159. [DOI] [PubMed] [Google Scholar]
- Csokasy J. Assessment of acute confusion: Use of the NEECHAM Confusion Scale. Applied Nursing Research. 1999;12(1):51–55. doi: 10.1016/s0897-1897(99)80189-x. [DOI] [PubMed] [Google Scholar]
- Culp K, Dyck M, Wakefield B, Cacchione, Decrane S, Decker SP. Bioimpedance analysis and other hydration parameters as risk factors for delirium in rural nursing home residents. The Journal of Gerontology: Medical Sciences. 2004;59A(8):813–817. doi: 10.1093/gerona/59.8.m813. [DOI] [PubMed] [Google Scholar]
- Culp K, Mentes J, Wakefield B. Hydration and acute confusion in long-term care residents. Western Journal of Nursing Research. 2003;25(3):251–266. doi: 10.1177/0193945902250409. [DOI] [PubMed] [Google Scholar]
- Davidson J, Getz M. Nutrition screening and assessment of anthropometry and bioelectrical impedance in the frail elderly: A clinical appraisal of methodology in a clinical setting. Journal of Nutrition in the Elderly. 2004;23(4):47–63. doi: 10.1300/J052v23n04_04. [DOI] [PubMed] [Google Scholar]
- Dharmarajan TS, Tankala H, Patel B, Sipalay M, Norkus EP. Outcome in ambulatory status immediately following hip fracture surgery in the acute setting: A comparison of nursing home residents and community older adults. Journal of the American Medical Directors Association. 2001;2(3):115–119. [PubMed] [Google Scholar]
- Di Iorio BR, Scalfi L, Terracciano V, Bellizzi V. A systematic evaluation of bioelectrical impedance measurement after hemodialysis session. Kidney International. 2004 Jun;65:2435–2440. doi: 10.1111/j.1523-1755.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- Dickson LR. Hypoalbuminemia in delirium. Psychosomatics. 1991;32(3):317–323. doi: 10.1016/S0033-3182(91)72071-9. [DOI] [PubMed] [Google Scholar]
- Evans BC, Crogan NL, Shultz JA. Resident coping strategies in the nursing home: An indicator of the need for dietary services change. Applied Nursing Research. 2004;17(2):109–115. doi: 10.1016/j.apnr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Fabiny AR, Kiel DP. Assessing and treating weight loss in nursing home patients. Clinics in Geriatric Medicine. 1997;13(4):737–751. [PubMed] [Google Scholar]
- Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: An overview of the state of the science. Journal of Gerontological Nursing. 2001;27(4):12. doi: 10.3928/0098-9134-20010401-06. [DOI] [PubMed] [Google Scholar]
- Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. Journal of the American Geriatrics Society. 2001;49(4):398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- Gessert CE, Calkins DR. Rural-urban differences in end-of-life care: the use of feeding tubes. Journal of Rural Health. 2001;17(1):16–24. doi: 10.1111/j.1748-0361.2001.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Gil Gregorio P, Ribera Casado JM. Dementia and nutrition: Intervention study in institutionalized patients. The Journal of Nutrition, Health, & Aging. 2003;7:304. [PubMed] [Google Scholar]
- Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clinics in Geriatric Medicine. 2002;18(4):737–757. doi: 10.1016/s0749-0690(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: a new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. 2003;51(1):96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- Holden M, Kelly C. Use of cholinesterase inhibitors in dementia. Advances in Psychiatric Treatment. 2002;8:89–96. [Google Scholar]
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. Journal of General Internal Medicine. 1998;13(4):234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, van Dyck C, Alessi C, Balkin S, Siegal A, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Irons MJ, Farace E, Brady WJ, Huff JS. Mental status screening of emergency department patients: normative study of the quick confusion scale. Academic Emergency Medicine. 2002;9(10):989–994. doi: 10.1111/j.1553-2712.2002.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Ely EW. The Confusion Assessment Method (CAM) International Journal of Geriatric Psychiatry. 2003;18(6):557–558. doi: 10.1002/gps.872. [DOI] [PubMed] [Google Scholar]
- Jagmin MG. Postoperative mental status in elderly hip surgery patients. Orthopedic Nursing. 1998;17(6):32–42. [PubMed] [Google Scholar]
- Jehan W, Nelson C. Advanced primary nursing: Liberating the talents. Nursing Management. 2006;12(9):20–33. doi: 10.7748/nm2006.02.12.9.20.c2042. [DOI] [PubMed] [Google Scholar]
- Johansson IS, Hamrin EK, Larsson G. Psychometric testing of the NEECHAM Confusion Scale among patients with hip fracture. Research in Nursing & Health. 2002;25(3):203–211. doi: 10.1002/nur.10036. [DOI] [PubMed] [Google Scholar]
- Kayser-Jones J. Inadequate staffing at mealtime. Implications for nursing and health policy. Journal of Gerontological Nursing. 1997;23(8):14–21. doi: 10.3928/0098-9134-19970801-07. [DOI] [PubMed] [Google Scholar]
- Keller HH, Hirdes JP. Using the minimum data set to determine the prevalence of nutrition problems in an Ontario population of chronic care patients. Canadian Journal of Dietary Practices Research. 2000;61(4):165–171. [PubMed] [Google Scholar]
- Lamarre CJ, Patten SB. A clinical evaluation of the neurobehavioral cognitive status examination in a general psychiatric inpatient population. Journal of Psychiatry & Neuroscience. 1994;19(2):103–108. [PMC free article] [PubMed] [Google Scholar]
- Laplante J, Cole MG. Detection of delirium using the confusion assessment method. Journal of Gerontological Nursing. 2001;27(9):16–23. doi: 10.3928/0098-9134-20010901-05. [DOI] [PubMed] [Google Scholar]
- Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Miller CK, Jensen GL. Dietary patterns of rural older adults are associated with weight and nutritional status. Journal of the American Geriatrics Society. 2004;52(4):589–595. doi: 10.1111/j.1532-5415.2004.52167.x. [DOI] [PubMed] [Google Scholar]
- Lesher EL, Berryhill JS. Validation of the Geriatric Depression Scale--Short Form among inpatients. Journal of Clinical Psychology. 1994;50(2):256–260. doi: 10.1002/1097-4679(199403)50:2<256::aid-jclp2270500218>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Levkoff S, Cleary P, Liptzin B, Evans DA. Epidemiology of delirium: An overview of research issues and findings. International Psychogeriatrics. 1991;3:149–167. doi: 10.1017/s1041610291000625. [DOI] [PubMed] [Google Scholar]
- Maitland LA, Myers ER, Hipp JA, Hayes WC, Greenspan SL. Read my hips: Measuring trochanteric soft tissue thickness. Calcified Tissue International. 1993;52(2):85–89. doi: 10.1007/BF00308313. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Matsushima E, Maruyama M. Early detection of postoperative delirium and confusion in a surgical ward using the NEECHAM confusion scale. General Hospital Psychiatry. 2004;26(2):158–163. doi: 10.1016/j.genhosppsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. Journal of General Internal Medicine. 2003;18(9):696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SL, Kiely DK, Gillick MR. Nursing home characteristics associated with tube feeding in advanced cognitive impairment. Journal of the American Geriatrics Society. 2003;51(1):75–79. [PubMed] [Google Scholar]
- Morris LM, MacIntyre RC, Redington JC. Pre- and posthemodialysis measures of serum albumin levels. American Nephrology Nurses Association Journal. 1998;25(2):231–234. [PubMed] [Google Scholar]
- Murray A. Enteral tube feeding: helping to provide nutritional support. Community Nurse. 2000;6(4):13–14. 17. [PubMed] [Google Scholar]
- Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: construction, validation, and clinical testing. Nursing Research. 1996;45(6):324–330. doi: 10.1097/00006199-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Nordenram G, Ljunggren G. Oral status, cognitive and functional capacity versus oral treatment need in nursing home residents: A comparison between assessments by dental and ward staff. Oral Disease. 2002;8(6):296–302. doi: 10.1034/j.1601-0825.2002.01788.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Yamagami H, Sawada S, Nakanishi M, Tamaki M, Ohnaka M, Sakamoto S, Niwa Y, Nakaya Y. The nutritional status of elderly bed-ridden patients receiving tube feeding. Journal of Nutritional Sciences and Vitaminology (Tokyo) 2001;47(3):236–241. doi: 10.3177/jnsv.47.236. [DOI] [PubMed] [Google Scholar]
- O'Neill D. The mini-mental status examination. Journal of the American Geriatrics Society. 1991;39(7):733. doi: 10.1111/j.1532-5415.1991.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Ortega RM, Requejo AM, Andres P, Lopez-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. American Journal of Clinical Nutrition. 1997;66(4):803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- Perry BA, Turner LW. A prediction model for polypharmacy: are older, educated women more susceptible to an adverse drug event? Journal of Women & Aging. 2001;13(4):39–51. doi: 10.1300/J074v13n04_04. [DOI] [PubMed] [Google Scholar]
- Piers LS, Soares MJ, Frandsen SL, O'Dea K. Indirect estimates of body composition are useful for groups but unreliable in individuals. International Journal of Obesity-Related Metabolic Disorders. 2000;24(9):1145–1152. doi: 10.1038/sj.ijo.0801387. [DOI] [PubMed] [Google Scholar]
- Pisani MA, Redlich C, McNicoll L, Ely EW, Inouye SK. Underrecognition of preexisting cognitive impairment by physicians in older ICU patients. Chest. 2003;124(6):2267–2274. doi: 10.1378/chest.124.6.2267. [DOI] [PubMed] [Google Scholar]
- Rapp CG, Onega LL, Tripp-Reimer T, Mobily P, Wakefield B, Kundrat M, Akins J, Wadle K, Mentes JC, Culp K, Meyer J, Waterman J. Training of acute confusion resource nurses: Knowledge, perceived confidence, and role. Journal of Gerontological Nursing. 2001;27(4):34–40. doi: 10.3928/0098-9134-20010401-08. [DOI] [PubMed] [Google Scholar]
- Requejo AM, Ortega RM, Robles F, Navia B, Faci M, Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. European Journal of Clinical Nutrition. 2003;57 Suppl 1:S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Montagnini M. Geriatric failure to thrive. American Family Physician. 2004;70(2):343–350. [PubMed] [Google Scholar]
- Rodriguez-Martin JL, Qizilbash N, Lopez-Arrieta JM. Thiamine for Alzheimer's disease. Cochrane Database System Review. 2001;(2):CD001498. doi: 10.1002/14651858.CD001498. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. The pathophysiology of wasting in the elderly. Journal of Nutrition. 1999;129 1S Suppl:256S–259S. doi: 10.1093/jn/129.1.256S. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Mahalanabis D. Empirically derived new equations for calculating body fat percentage based on skinfold thickness and midarm circumference in preschool Indian children. American Journal of Human Biology. 2004;16(3):278–288. doi: 10.1002/ajhb.20030. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA, Brooks JO, 3rd, Friedman L, Gratzinger P, Hill RD, Zadeik A, Crook T. Proposed factor structure of the Geriatric Depression Scale. International Psychogeriatrics. 1991;3(1):23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- Sheiman SL, Pomerantz JD. Tube feeding in dementia: a controversial practice. Journal Nutrition Health and Aging. 1998;2(3):184–189. [PubMed] [Google Scholar]
- Silver HJ, Wellman NS, Arnold DJ, Livingstone AS, Byers PM. Older adults receiving home enteral nutrition: Enteral regimen, provider involvement, and health care outcomes. Journal Parenteral & Enteral Nutrition. 2004;28(2):92–98. doi: 10.1177/014860710402800292. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Adams FA, Groccia ME, DeVeaux R, Growdon JH, Pendlebury WW. Correlational analysis of five commonly used measures of mental status/functional abilities in patients with Alzheimer disease. Alzheimer Disease & Associated Disorders. 1999;13(3):147–150. doi: 10.1097/00002093-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Strub RL, Black FW. The Mental Status Examination in Neurology. 2nd ed. Philadelphia: F.A. Davis; 1985. [Google Scholar]
- Tai ES, Ho SC, Fok AC, Tan CE. Measurement of obesity by anthropometry and bioelectric impedance analysis: Correlation with fasting lipids and insulin resistance in an Asian population. Annals Academy of Medicine Singapore. 1999;28(3):445–450. [PubMed] [Google Scholar]
- van Vliet MJ, Schuurmans MJ, Grypdonck MH, Duijnstee MS. Improper intake of medication by elders--insights on contributing factors: A review of the literature. Research & Theory for Nursing Practice. 2006;20(1):79–93. doi: 10.1891/rtnp.20.1.79. [DOI] [PubMed] [Google Scholar]
- VanderJagt DJ, Harmatz P, Scott-Emuakpor AB, Vichinsky E, Glew RH. Bioelectrical impedance analysis of the body composition of children and adolescents with sickle cell disease. Journal of Pediatrics. 2002;140(6):681–687. doi: 10.1067/mpd.2002.124385. [DOI] [PubMed] [Google Scholar]
- Volpato S, Romagnoni F, Soattin L, Ble A, Leoci V, Bollini C, Fellin R, Zuliani G. Body mass index, body cell mass, and 4-year allcause mortality risk in older nursing home residents. Journal of the American Geriatrics Society. 2004;52(6):886–891. doi: 10.1111/j.1532-5415.2004.52254.x. [DOI] [PubMed] [Google Scholar]
- Wendland BE, Greenwood CE, Weinberg I, Young KW. Malnutrition in institutionalized seniors: the iatrogenic component. Journal of the American Geriatrics Society. 2003;51(1):85–90. doi: 10.1034/j.1601-5215.2002.51015.x. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk The Framingham experience. Archives Internal Medicine. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]