Fig. 5.
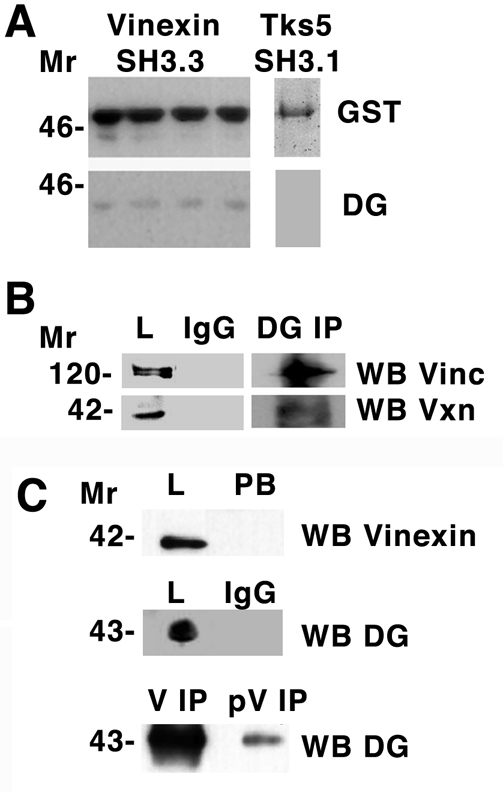
Dystroglycan interacts with vinculin and vinexin. (A) Total cell lysate from myoblasts was applied to a glutathione-Sepharose column with bound third SH3 domain of vinexin fused to GST. Following extensive washing, GST-SH3 was eluted with reduced glutathione and fractions western blotted (WB) for GST or dystroglycan (DG). Dystroglycan was specifically retained by the GST-SH3 column. A control SH3, the first SH3 domain of Tks5, did not interact with dystroglycan in a similar but batch bound experiment. (B) Immunoprecipitation (IP) of dystroglycan or control IgG from myoblast lysates followed by western blotting (WB) with antibodies that recognise vinculin (Vinc) or vinexin-β (Vxn). Vinculin or vinexin-β are recognised in the total lysate (L) fraction in the dystroglycan IP, but not when control IgG is used in the IP. (C) Dystroglycan is also identified by western blotting in immunoprecipitations carried out with either anti-vinexin-β (V) or anti-phosphorylated vinexin-β (pV); however, the amount of dystroglycan recovered in the anti-phosphorylated vinexin-β IP is considerably less. Anti-vinexin-β western blot of total lysate (L) and post binding (PB) fractions indicate that the anti-vinexin-β antibody completely depletes the lysate, whereas control IgG immunoprecipitations do not pull down any dystroglycan. Molecular size markers (in kDa) are shown on the left.
