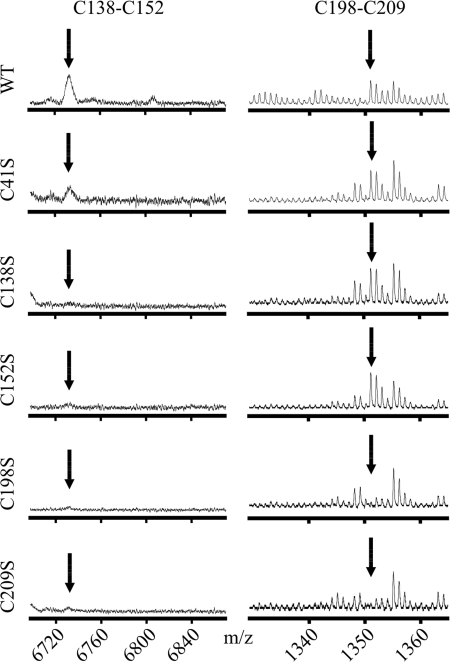
FIGURE 7.
Disulfide bond formation in mutant GlyRα1 ECDs. MALDI-TOF mass spectra of unmodified tryptic fragments of different GlyRα1 ECD variants refolded under oxidative conditions. The signals corresponding to the Cys-loop (left, Cys138–Cys152) and GlyR-specific disulfide bond (right, Cys198–Cys209) are shown (see Figs. 2 and 3 for reference). Mutant C41S shows unaltered cysteine bond formation as compared with the wild-type domain (WT). As expected, for mutants C138S and C152S the signal for Cys198–Cys209 but not Cys138–Cys152 is observed. In contrast, mutants C198S and C209S display no signal for disulfide bond formation, indicating the requirement of the GlyR-specific for the effective formation of the Cys-loop disulfide bond in vitro.