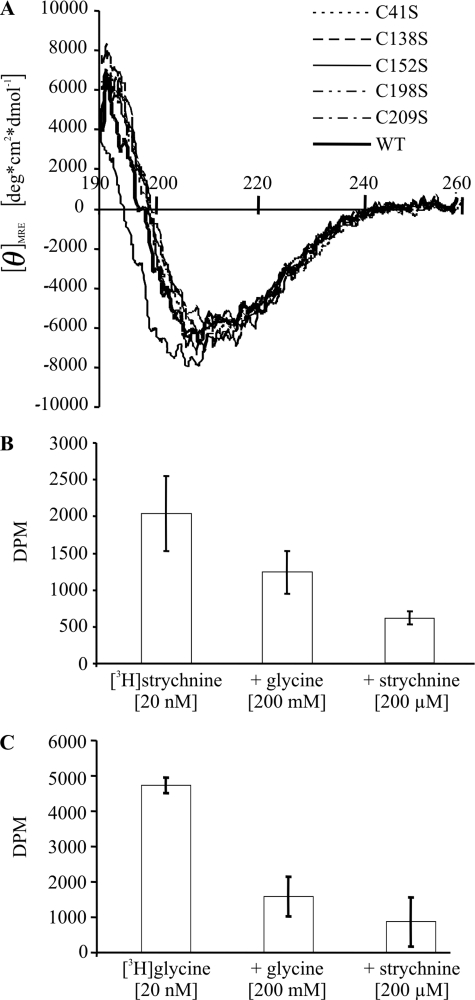
FIGURE 8.
A, circular dichroism spectra of refolded wild-type (WT) and cysteine mutant GlyRα1 ECDs. Superimposition of CD spectra of wild-type and cysteine mutant ECDs indicates an overall comparable content of secondary structure. B and C, binding (±S.D.) of 20 nm [3H]strychnine (B) and 20 nm [3H]glycine (C) to refolded wild-type GlyRα1 ECD. Binding of both radioligands was inhibited by an excess of either unlabeled glycine or strychnine. For the GlyRα1 ECD preparation shown, the KD for [3H]strychnine was 25 nm.