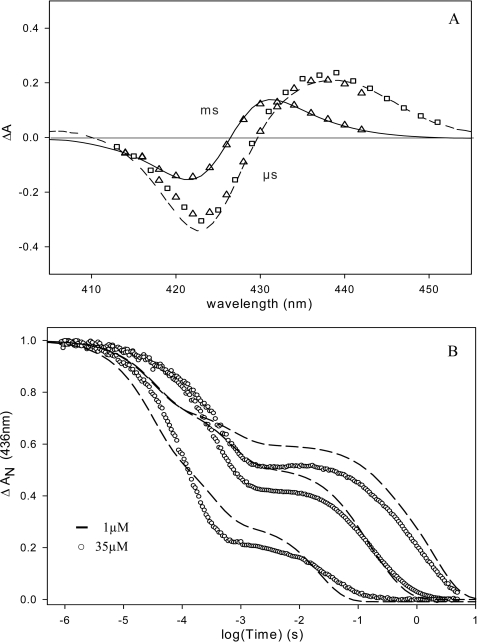
FIGURE 4.
A, transient absorption spectra after flash photolysis of the heme-CO complex of EcDos (▵) and EcDosH (□) at 0. 1 μs and ms time scales. – – –, transient spectrum at 4 ns; —, difference between steady-state spectra: deoxy hexacoordinated His-Fe2+-Met minus His-Fe2+-CO. B, flash photolysis kinetics for EcDosH at 436 nm and 25 °C. Recombination kinetics at different CO concentrations, from top to bottom: 0.01, 0.1, and 1 atm of CO. After flash photolysis of CO, the first phase represents a competitive binding between CO and Met to the heme. The second phase is a slow replacement reaction of Met by CO to return to the preflash steady state. Heme concentration dependence on CO kinetics is observed, due to the presence of two protein structures in equilibrium: monomeric and dimeric with different Met on- and off-rates.