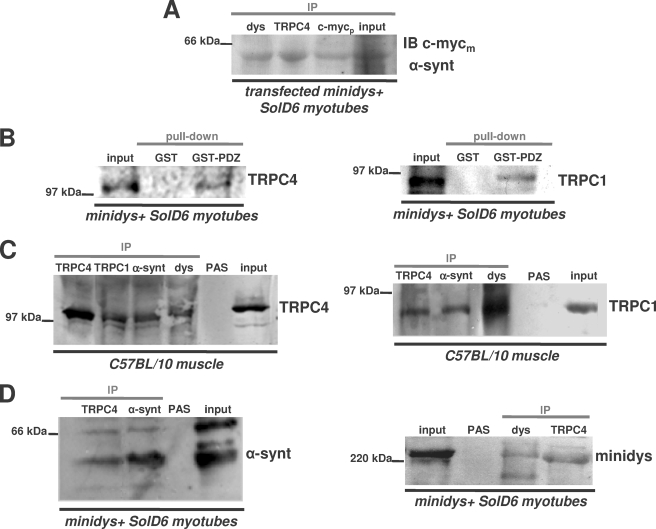
FIGURE 6.
TRPC4 associates in vitro with the PDZ domain of α1-syntrophin and forms a macromolecular complex with TRPC1, dystrophin, and α1-syntrophin in skeletal muscle and cultured myotubes. A, the lysates from differentiated minidys+ SolD6 myotubes transfected with FL-α1-syntrophin-c-Myc-EGFP plasmid were immunoprecipitated (IP) with polyclonal antibodies against c-Myc tag (c-Mycp lane), TRPC4 (TRPC4 lane), and dystrophin (dys lane). The resulting immunoblot (IB) was probed with a monoclonal antibody against c-Myc tag (c-Mycm). B, beads charged with GST alone or with a GST fusion protein of the PDZ domain of α1-syntrophin were incubated with protein lysates from minidys+ SolD6 myotubes. Bound proteins were immunoblotted with a polyclonal TRPC4 antibody or with a polyclonal TRPC1 antibody. The input lane was loaded with 10% of the extract used for the pulldown. C, muscle lysates from C57BL/10 muscles (input lane) were incubated with polyclonal antibodies against dystrophin (dys lane), α1-syntrophin (α-synt lane), TRPC1 (TRPC1 lane), and TRPC4 (TRPC4 lane). Western blots of the immunoprecipitated proteins were probed with polyclonal antibodies against TRPC4 or TRPC1. PAS, protein A-Sepharose. D, protein lysates (input lane) from minidys+ SolD6 myotubes were incubated with polyclonal antibodies against α1-syntrophin, TRPC4, and mini-dystrophin (dys lane). Western blots of the immunoprecipitated proteins were probed with polyclonal antibodies against α1-syntrophin or mini-dystrophin.