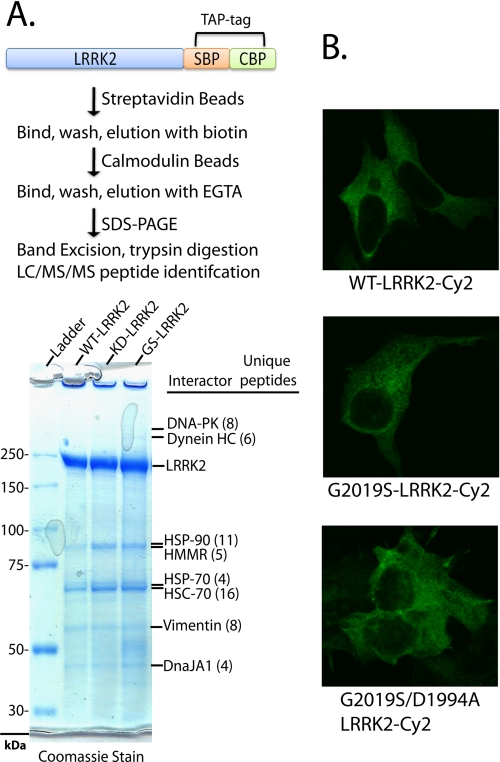
FIGURE 2.
LRRK2 protein interactions and subcellular distribution are independent of LRRK2-kinase activity. A, LRRK2-positive protein complexes were purified from native lysates derived from HEK-293FT cells transfected with WT-LRRK2, kinase-dead (D1994A)-LRRK2, or kinase-overactive G2019S-LRRK2 constructs, through a tandem affinity purification strategy as illustrated. LRRK2 and interacting proteins were resolved from affinity beads, analyzed on a Tris acetate SDS gel, and stained with Coomassie dye, and the constituency of individual protein bands was identified by mass spectrometry. All unique proteins with more than two unique peptide hits of high quality are indicated, and in every case identified proteins matched the expected size in reference to the protein ladder. B, confocal microscopic analysis of HEK-293T cells transfected with the indicated plasmid. Cells were fixed, and LRRK2 expression was detected with anti-Myc-Cy2 antibody (shown as green signal), and cell images are representative. SBP, streptavidin-binding peptide; CBP, calmodulin-binding peptide.