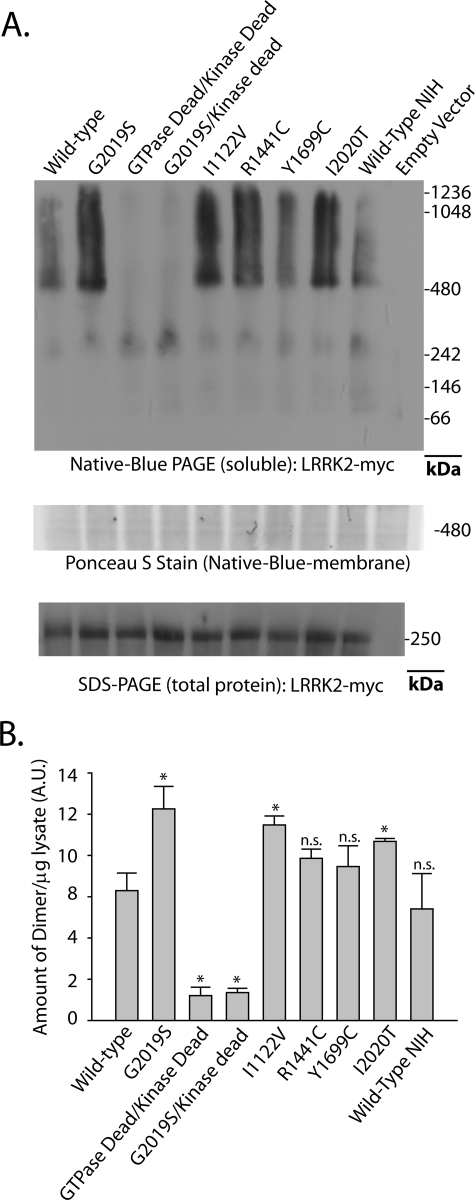
FIGURE 3.
PD-associated LRRK2 mutations enhance the proportion of soluble LRRK2 dimer-sized and high molecular weight species. A, HEK-293T cells were transfected with constructs encoding human LRRK2 that harbors the indicated mutation, where GTPase-dead is K1347A-LRRK2 and kinase-dead is D1994A-LRRK2. A vector encoding WT-LRRK2 (Wild Type-NIH) derived independently from LRRK2-vectors used in this study was provided by Mark Cookson. Cell pellets were split equally for lysis by freeze/thaw cycles directly in PBS or lysis with 1% SDS and PBS with sonication, and 10 μg of protein lysate (as determined by BCA protein assay) was loaded onto a native gel (3–12% bis-tris) or an SDS gel (7% Tris acetate-SDS), respectively. Ponceau S stain was applied to PVDF membranes after transfer of protein complexes to PVDF to ensure even transfer, and the region near 500 kDa is shown and demonstrates the presence of protein across the membrane. LRRK2 complexes were visualized with the anti-c-Myc antibody by Western blot and imaged on a LI-COR Odyssey. Western blots representative of five independent experiments are shown. B, normalization of the LRRK2 dimer-sized structure (signal from ∼480 to ∼550 kDa) to total LRRK2 protein (SDS-solubilized) using densitometry analysis. Error bars, ±S.E. *, p < 0.009. n.s., nonsignificant in comparison with wild-type LRRK2, assessed by unpaired Student's t test.