FIGURE 5.
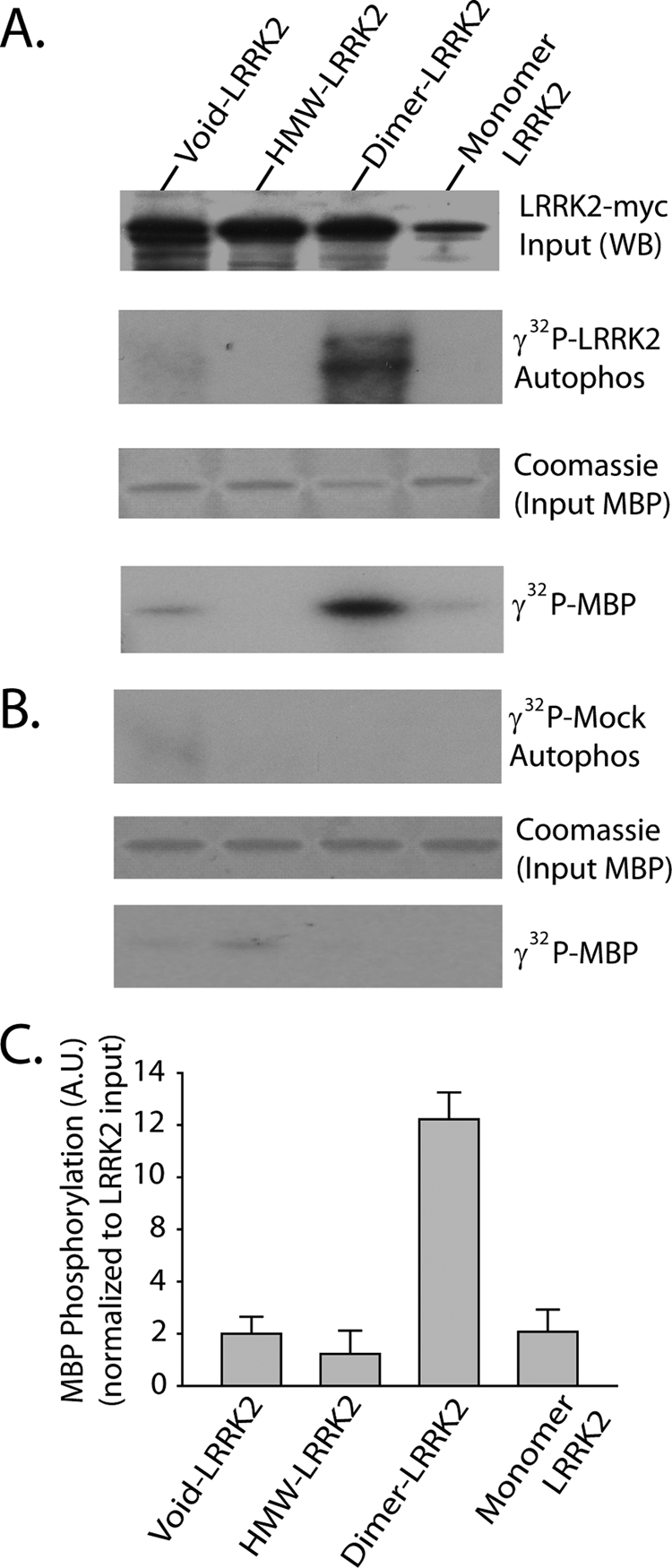
Dimer-sized G2019S-LRRK2 complexes are kinase-active conformations. A, total soluble native protein lysates derived from cells expressing G2019S-LRRK2 were separated with a Superdex 200 10/300 GL column. LRRK2 protein complexes from fractions 5 (void), 8 (dimer), or 10 (monomer) were immunoprecipitated with magnetic beads conjugated to anti-Myc antibody and combined into a kinase reaction containing dephosphorylated MBP protein that serves as an efficient LRRK2 substrate in vitro. Supernatant containing MBP was analyzed by SDS-PAGE and Coomassie stain. Eluted G2019S-LRRK2 protein was resolved onto SDS-gels, transferred to PVDF, and exposed to autoradiography film, and input levels from kinase reactions were determined with anti-Myc-HRP antibody. B, mock kinase reactions using fractionated lysate and immunoprecipitates from cells transected with empty vector and autoradiography films exposed for identical times as in A. C, quantification of LRRK2-mediated MBP phosphorylation via densitometry, adjusted for background and normalized to relative input levels of LRRK2 protein. Data are derived from three independent experiments, and error bars represent ±S.E. A.U., arbitrary units.
