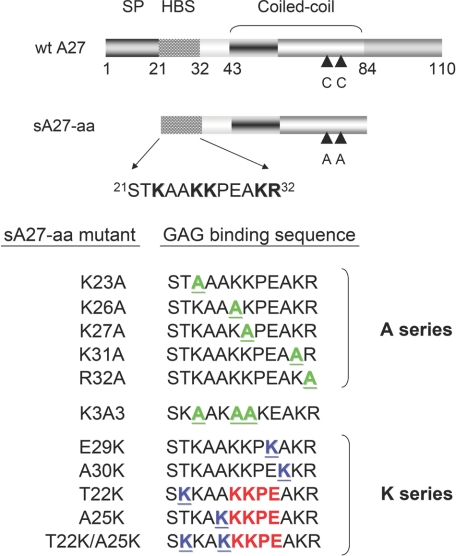
FIGURE 1.
Illustration of the different functional domains in wild-type A27 and its truncated form sA27-aa (amino acids 21–84). Wild type A27 (wt A27) consists of the four functional domains of the signal peptide (SP, residues 1–20), the heparin binding domain (residues 21–32), the coiled-coil rigid domain (residues 43–84), and the Leu zipper domain at the C terminus (residues 84–110). sA27-aa consists of the heparin binding domain and the coiled-coil rigid domain in which two adjacent Cys residues (CC) at residues 71 and 72 are mutated to Ala. The HBS is an Arg/Lys-rich flexible domain, STKAAKKPEAKR; the basic residues are indicated in boldface. Different HBS mutants (A and K series and the K3A3 mutant) were expressed and purified; the mutated residues are shown in green (A series) or blue (K series) and are underlined. The KKPE-binding motif in the K mutants is shown in red.