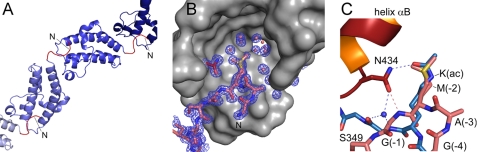
FIGURE 6.
Binding of the N-terminal linker sequence GAMG-S349KI to Brd4 BD2. A, in the protein crystal BD2 bromodomains are successively linked by their N-terminal tail sequence GAMGS (colored red), which leads to formation of continuous strings. B, electron density omit map of the bromodomain binding site for Nζ-acetyl-lysines contoured at 1 σ. The methionine side chain sticks into the binding pocket for acetylated lysines of a neighboring molecule and interacts with Asn-434 of Brd4 BD2. The terminal methyl group (Cϵ) of methionine adopts two different conformations with an approximate 6:4 population ratio. The moiety is surrounded by a shell of water molecules at the base of the recognition cavity. C, superimposition of the N-terminal linker sequence GAMGS bound to its proximate bromodomain from Brd4 BD2 (red) with the complex of a histone H4 tail peptide comprising acetylated lysine 16 with the bromodomain Gcn5 (blue; 1E6I; Ref. 35). The hydrogen bond network is displayed as dashed lines. Note that the backbone atoms of the GAMG sequence are roped into the binding pocket leading to the formation of a direct hydrogen bond between Asn-434 and the G(-1) amide group that is otherwise mediated by a water molecule upon binding to acetylated lysines.