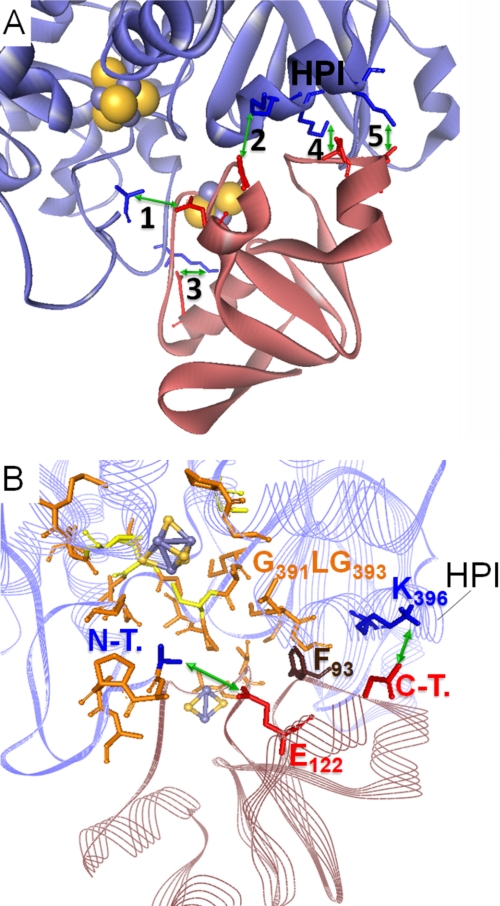
FIGURE 5.
Model of the PetF-HydA1 electron transfer complex derived from the SDM effect-based screening of possible in silico docking complexes. A, zoomed view on the model complex interface with five possible salt bridge contacts (green arrows) between conserved residues of PetF (light red ribbon model) and HydA1 (blue ribbon model). 1, N-terminal amino group of HydA1-Ala57⇔PetF-Glu122; 2, HydA1-Glu396*⇔C-terminal carboxyl group of PetF-Tyr126; 3, HydA-Lys433⇔PetF-Asp56; 4, HydA1-Lys397*⇔PetF-Asp95; 5, HydA1-Lys501*⇔PetF-Asp96. Residues marked with * are localized within a single α-helix that is especially conserved in green algal hydrogenases and termed HPI in this study. B, integration of PetF into the putative binding side on HydA1 near the active center that is enclosed by hydrophobic residues. Hydrophobic residues probably participating in complex stability are depicted as orange stick models. N-T., N-terminal amino group of HydA1; C-T., C-terminal carboxyl group of PetF.