Abstract
Mapatumumab (HGS-ETR1) is a fully human IgG1 agonistic monoclonal antibody that exclusively targets and activates tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1). It was tested in vitro at concentrations from 0.01 to 100 μg/ml and in vivo at a dose of 10 mg/kg administered intraperitoneally using a twice-weekly schedule. Mapatumumab demonstrated limited activity against the 23 cell lines of the PPTP in vitro panel with no lines achieving 50% growth inhibition. Mapatumumab induced significant differences in event-free survival distribution compared to controls in 9 of 37 evaluable solid tumor xenografts tested, but in none of the 8 ALL xenografts.
Keywords: Preclinical Testing, Developmental Therapeutics, Mapatumumab
INTRODUCTION
Mapatumumab (HGS-ETR1) is a fully human IgG1 agonistic monoclonal antibody that exclusively targets and activates tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1). Mapatumumab induces regressions as a single agent against TRAIL-R1–expressing adult tumor xenografts of multiple histologies (e.g., colon, non-small cell lung, and renal cancer) [1], and it enhances the antitumor activity of cytotoxic agents against multiple adult cancer cell lines [1,2]. Mapatumumab is under clinical evaluation in adults with cancer, both as a single agent and in combination with cytotoxic agents [3,4].
Previous reports have described TRAIL-induced apoptosis in pediatric cell lines. For example, in a panel of 7 rhabdomyosarcoma cell lines, three showed high level sensitivity to TRAIL [5]. TRAIL-induced apoptosis has also been reported to occur in a high percentage of Ewing sarcoma cell lines, and both Ewing sarcoma cell lines and clinical specimens express TRAIL-R1 and TRAIL-R2 in a high percentage of cases [6–8]. Neuroblastoma cell lines are generally reported to be resistant to TRAIL-induced apoptosis [9–11], which may be the result of lack of caspase-8 expression secondary to promoter methylation as well as due to the absence of both TRAIL-R1 and TRAIL-R2 in some cell lines [9–11]. The PPTP evaluated mapatumumab to gain insight into the potential of TRAIL-R1 directed therapy for pediatric tumors.
MATERIALS AND METHODS
In vitro testing
In vitro testing was performed using the DIMSCAN method, as previously described [12]. Cells were incubated in the presence of mapatumumab for 96 hours at concentrations from 0.01 μg/ml to 100 μg/ml and analyzed as previously described[13].
In vivo tumor growth inhibition studies
CB17SC-M scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [14,15]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [16]. Experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Responses were determined using three activity measures as previously described [17]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
Mapatumumab was provided to the Pediatric Preclinical Testing Program by Human Genome Sciences. Mapatumumab was dissolved in phosphate buffered saline and administered intraperitoneally using a twice-weekly schedule for 6 weeks at a dose of 10 mg/kg. Mapatumumab was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
In vitro testing
Mapatumumab was tested against the PPTP’s in vitro cell lines at concentrations ranging from 0.01 μg/ml to 100 μg/ml. Mapatumumab demonstrated very limited activity against the 23 cell lines of the PPTP in vitro panel, with no lines achieving 50% growth inhibition. The minimum T/C (%) values for each cell line tested are provided in Supplemental Table I.
In vivo testing
Mapatumumab was evaluated in 46 xenograft models and was well tolerated at the dose and schedule used for in vivo testing. For unknown reasons, two of the neuroblastoma xenografts (NB-1643 and NB-SD) showed excessive toxicity (15 of 20 toxic deaths) when initially tested against mapatumumab. Repeat testing of the same xenografts produced no toxicity. With the initial testing of the two neuroblastoma xenografts omitted, treated and control animals experienced similar toxicity rates with 12 of 894 (1.3%) mice dying during the study [5 of 434 (1.2%) in the control arms and 7 of 440 (1.6%) in the mapatumumab treatment arms]. There were 45 xenograft models evaluable for efficacy, with only one xenograft line (NB-1771) excluded from reporting because of excessive toxicity. A complete summary of results is provided in Supplemental Table II, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
Mapatumumab induced significant differences in EFS distribution compared to controls in 9 of 37 evaluable solid tumor xenografts tested (Table I). Significant differences in EFS distribution occurred in one-half of xenografts in the glioblastoma panel (2 of 4) and the osteosarcoma panel (3 of 6). None of the 8 ALL xenografts demonstrated significant differences in EFS distribution between the treated and control groups. Although there were significant differences in EFS distribution for selected solid tumor xenografts, the EFS T/C values were below the criteria for intermediate activity for the time to event measure of activity (EFS T/C > 2). No objective responses were observed in any of the solid tumor panels or in the ALL panel. The best response was PD2 (progressive disease with growth delay), with PD2 activity concentrated in the glioblastoma panel (2 of 4) and the neuroblastoma panel (2 of 5) (Table I). The objective response results for both solid tumors and leukemia models in a ‘COMPARE’ format, based on the objective response scoring criteria centered around the midpoint score of 5 that represents stable disease (Supplemental Figure 1).
Table I.
Activity for Mapatumumab against the PPTP in vivo
| Xenograft Line |
Histology | KM Estimate of Median Time to Event |
P-value | EFS T/C | Median Final RTV |
Tumor Volume T/C |
P-Value | Median Group Response |
T/C Activity |
EFS Activity |
Response Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | 14.7 | 0.302 | 0.8 | >4 | 1.11 | 0.315 | PD1 | Low | Low | Low |
| KT-14 | Rhabdoid | 18.2 | 0.409 | 1 | >4 | 0.85 | 0.661 | PD1 | Low | Low | Low |
| KT-12 | Rhabdoid | 11.5 | 0.287 | 1 | >4 | 0.86 | 0.529 | PD1 | Low | Low | Low |
| KT-10 | Wilms | 8.5 | 0.174 | 0.8 | >4 | 1.1 | 0.497 | PD1 | Low | Low | Low |
| KT-11 | Wilms | 11.8 | 0.551 | 1.1 | >4 | 0.87 | 0.442 | PD1 | Low | Low | Low |
| KT-13 | Wilms | 8.9 | 0.788 | 0.9 | >4 | 1.11 | 0.549 | PD1 | Low | Low | Low |
| SK-NEP-1 | Ewing | 8.8 | <0.001 | 1.1 | >4 | 0.88 | 0.218 | PD1 | Low | Low | Low |
| EW5 | Ewing | 15.9 | 0.217 | 1.2 | >4 | 0.98 | 0.447 | PD1 | Low | Low | Low |
| EW8 | Ewing | 14.6 | 0.094 | 1.2 | >4 | 0.88 | 0.123 | PD1 | Low | Low | Low |
| TC-71 | Ewing | 8 | 0.64 | 1 | >4 | 1.05 | 0.897 | PD1 | Low | Low | Low |
| CHLA258 | Ewing | 11 | 0.147 | 0.8 | >4 | 1.68 | 0.052 | PD1 | Low | Low | Low |
| Rh28 | ALV RMS | 23.9 | 0.67 | 1.1 | >4 | 0.67 | 0.143 | PD1 | Low | Low | Low |
| Rh30 | ALV RMS | 20.1 | 0.657 | 1 | >4 | 1.26 | 0.739 | PD1 | Low | Low | Low |
| Rh30R | ALV RMS | 15.6 | 0.423 | 1 | >4 | 0.91 | 0.393 | PD1 | Low | Low | Low |
| Rh41 | ALV RMS | 12.4 | 0.014 | 0.9 | >4 | 1.12 | 0.016 | PD1 | Low | Low | Low |
| Rh65 | ALV RMS | 21.7 | 0.29 | 0.9 | >4 | 1.14 | 0.360 | PD1 | Low | Low | Low |
| Rh18 | EMB RMS | 12.5 | 0.07 | 1.1 | >4 | 0.77 | 0.133 | PD1 | Low | Low | Low |
| BT-28 | Medulloblastoma | 11.9 | 0.724 | 1 | >4 | 1.02 | 1.000 | PD1 | Low | Low | Low |
| BT-45 | Medulloblastoma | 18.3 | <0.001 | 1.2 | >4 | 0.67 | <0.001 | PD1 | Low | Low | Low |
| BT-50 | Medulloblastoma | > EP | 0.347 | > 1.0 | 3.8 | 0.76 | 0.029 | PD2 | Low | NE | Int |
| BT-36 | Ependymoma | > EP | 0.591 | . | 2.8 | 0.95 | 1.000 | PD2 | Low | NE | Int |
| BT-44 | Ependymoma | 13.4 | 0.085 | 0.9 | >4 | 1.28 | 0.247 | PD1 | Low | Low | Low |
| GBM2 | Glioblastoma | 34.2 | 0.001 | 1.8 | >4 | 0.54 | <0.001 | PD2 | Low | Low | Int |
| BT-39 | Glioblastoma | 11.4 | 0.858 | 1 | >4 | 1.14 | 0.393 | PD1 | Low | Low | Low |
| D645 | Glioblastoma | 10.6 | 0.809 | 1 | >4 | 0.96 | 0.780 | PD1 | Low | Low | Low |
| D456 | Glioblastoma | 10.2 | 0.002 | 1.7 | >4 | 0.58 | 0.004 | PD2 | Low | Low | Int |
| NB-1691 | Neuroblastoma | 10.3 | 0.134 | 0.8 | >4 | 1.65 | 0.029 | PD1 | Low | Low | Low |
| NB-EBc1 | Neuroblastoma | 26.3 | <0.001 | 1.6 | >4 | 0.37 | 0.006 | PD2 | Int | Low | Int |
| CHLA-79 | Neuroblastoma | 6.7 | 0.22 | 0.8 | >4 | 1.33 | 0.393 | PD1 | Low | Low | Low |
| NB-SD | Neuroblastoma | 20.1 | 0.051 | 1.6 | >4 | 0.58 | 0.075 | PD2 | Low | Low | Int |
| NB-1643 | Neuroblastoma | 30.2 | 0.005 | 1.2 | >4 | 0.81 | 0.968 | PD1 | Low | Low | Low |
| OS-1 | Osteosarcoma | 33.7 | 0.527 | 1.1 | >4 | 0.9 | 0.353 | PD1 | Low | Low | Low |
| OS-2 | Osteosarcoma | 23 | <0.001 | 1.3 | >4 | 0.67 | 0.002 | PD1 | Low | Low | Low |
| OS-17 | Osteosarcoma | 25.9 | 0.021 | 1.3 | >4 | 0.7 | 0.028 | PD1 | Low | Low | Low |
| OS-9 | Osteosarcoma | > EP | 0.001 | > 1.5 | 3.7 | 0.63 | <0.001 | PD2 | Low | NE | Int |
| OS-33 | Osteosarcoma | 14.1 | 0.244 | 1.1 | >4 | 0.83 | 0.353 | PD1 | Low | Low | Low |
| OS-31 | Osteosarcoma | 22 | 0.473 | 1.4 | >4 | 0.67 | 0.052 | PD1 | Low | Low | Low |
| ALL-2 | ALL B-precursor | 18.4 | 0.786 | 1.2 | >25 | . | PD1 | Low | Low | ||
| ALL-3 | ALL B-precursor | 6.1 | 0.028 | 0.5 | >25 | . | PD1 | Low | Low | ||
| ALL-4 | ALL B-precursor | 1 | 0.2 | 1 | >25 | . | PD1 | Low | Low | ||
| ALL-7 | ALL B-precursor | 4.1 | 0.68 | 2 | >25 | . | PD2 | Low | Int | ||
| ALL-8 | ALL T-cell | 8 | 0.315 | 1 | >25 | . | PD1 | Low | Low | ||
| ALL-16 | ALL T-cell | 11.3 | 0.5 | 1.1 | >25 | . | PD1 | Low | Low | ||
| ALL-17 | ALL B-precursor | 5.2 | 0.467 | 1 | >25 | . | PD1 | Low | Low | ||
| ALL-19 | ALL B-precursor | 11.8 | 0.458 | 1.2 | >25 | . | PD1 | Low | Low |
DISCUSSION
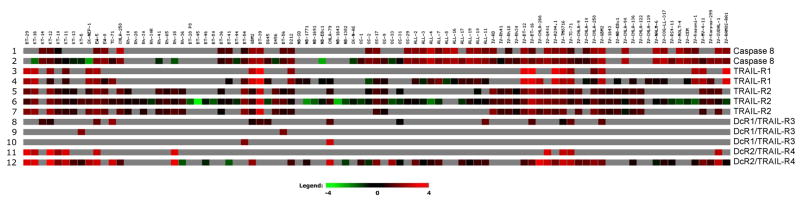
Mapatumumab demonstrated limited activity against the PPTP in vitro cell line panels and against its in vivo xenograft tumor panels. The limited activity observed for mapatumumab against the PPTP’s pediatric preclinical models could result from multiple mechanisms [18], including: lack of TRAIL-R1 expression, which is noted at the RNA level for multiple PPTP xenografts and cell lines (Figure 1); inactivation of proapoptotic Bcl-2 family proteins (e.g., Bax gene deletions) [19] or overexpression of anti-apoptotic Bcl-2 family proteins; expression of XIAP; and loss of caspase-8 expression. The latter mechanism may be particularly relevant to pediatric cancers such as neuroblastoma and Ewing sarcoma, for which caspase-8 down-regulation has been associated with TRAIL-resistance and for which caspase-8 re-expression has been associated with restored TRAIL responsiveness [9–11]. Low expression of caspase-8 is observed for several PPTP panels, most notably the neuroblastoma panel (Figure 1). The limited activity of mapatumumab against the PPTP in vivo models is unlikely to be due to failure to achieve effective systemic exposures, as mapatumumab at the dose and schedule used by the PPTP showed substantial preclinical activity against selected adult cancer models [1]. Additionally, the systemic exposures achieved for the dose and schedule used by the PPTP are comparable to or exceed those observed in adults receiving mapatumumab at the recommended phase 2 dose [1,3,4].
Figure 1.
Gene expression analysis for selected genes related to TRAIL pathway signaling using the Affymetrix HG-U133Plus2 GeneChip (54,613 probesets) as previously described [23]
There are several options to pursue in terms of further preclinical studies focused on developing TRAIL-directed therapies in the pediatric setting. For example, TRAIL-R2 targeted agents (e.g., lexatumumab) or approaches that engage both TRAIL-R1 and TRAIL-R2 (e.g., combined use of mapatumumab and lexatumumab or use of recombinant human Apo2L/TRAIL) could be explored. Expression of TRAIL-R2 is somewhat more common than TRAIL-R1 in the PPTP xenografts and cell lines at the RNA level (Figure 1), and previous work has suggested that the response of rhabdomyosarcoma cell lines to TRAIL is through TRAIL-R2 [5]. Another option for future work is evaluating a TRAIL pathway targeted agent such as mapatumumab or lexatumumab in combination with cytotoxic chemotherapy. Preclinical studies of these agents in adult cancer models support this strategy [20], as does previous work using sarcoma cell lines demonstrating that anticancer agents (e.g., cisplatin, doxorubicin) can reduce levels of FLIP and/or XIAP, thereby sensitizing the cell lines to the apoptosis-inducing effects of TRAIL signaling pathway activation [21,22]. Activity leads identified through future testing could help guide clinical development of TRAIL-directed therapies in children with cancer.
Supplementary Material
Supplemental Figure 1. Representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute, and mapatumumab was provided by Human Genome Sciences. In addition to the authors represents work contributed by the following: Sherry Ansher, Joshua Courtright, Edward Favours, Henry S. Friedman, Debbie Payne-Turner, Charles Stopford, Chandra Tucker, Jianrong Wu, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
References
- 1.Pukac L, Kanakaraj P, Humphreys R, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92(8):1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgakis GV, Li Y, Humphreys R, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130(4):501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 3.Hotte SJ, Hirte HW, Chen EX, et al. A Phase 1 Study of Mapatumumab (Fully Human Monoclonal Antibody to TRAIL-R1) in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2008;14(11):3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 4.Tolcher AW, Mita M, Meropol NJ, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25(11):1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 5.Petak I, Douglas L, Tillman DM, et al. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6(10):4119–4127. [PubMed] [Google Scholar]
- 6.Mitsiades N, Poulaki V, Mitsiades C, et al. Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61(6):2704–2712. [PubMed] [Google Scholar]
- 7.Kumar A, Jasmin A, Eby MT, et al. Cytotoxicity of Tumor necrosis factor related apoptosis-inducing ligand towards Ewing’s sarcoma cell lines. Oncogene. 2001;20(8):1010–1014. doi: 10.1038/sj.onc.1204154. [DOI] [PubMed] [Google Scholar]
- 8.Van Valen F, Fulda S, Truckenbrod B, et al. Apoptotic responsiveness of the Ewing’s sarcoma family of tumours to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) Int J Cancer. 2000;88(2):252–259. doi: 10.1002/1097-0215(20001015)88:2<252::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Eggert A, Grotzer MA, Zuzak TJ, et al. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 2001;61(4):1314–1319. [PubMed] [Google Scholar]
- 10.Yang X, Merchant MS, Romero ME, et al. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63(5):1122–1129. [PubMed] [Google Scholar]
- 11.Yang X, Thiele CJ. Targeting the tumor necrosis factor-related apoptosis-inducing ligand path in neuroblastoma. Cancer Lett. 2003;197(1–2):137–143. doi: 10.1016/s0304-3835(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 12.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 13.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.21214. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 15.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 16.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 17.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12(3):228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8(3):274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys RC, Halpern W. Trail receptors: targets for cancer therapy. Adv Exp Med Biol. 2008;615:127–158. doi: 10.1007/978-1-4020-6554-5_7. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita H, Yoshikawa H, Shiiki K, et al. Cisplatin (CDDP) sensitizes human osteosarcoma cell to Fas/CD95-mediated apoptosis by down-regulating FLIP-L expression. Int J Cancer. 2000;88(6):986–991. doi: 10.1002/1097-0215(20001215)88:6<986::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Mirandola P, Sponzilli I, Gobbi G, et al. Anticancer agents sensitize osteosarcoma cells to TNF-related apoptosis-inducing ligand downmodulating IAP family proteins. Int J Oncol. 2006;28(1):127–133. [PubMed] [Google Scholar]
- 23.Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14(14):4572–4583. doi: 10.1158/1078-0432.CCR-07-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.