Figure 1. RGS14 binds and co-localizes with activated H-Ras in cells.
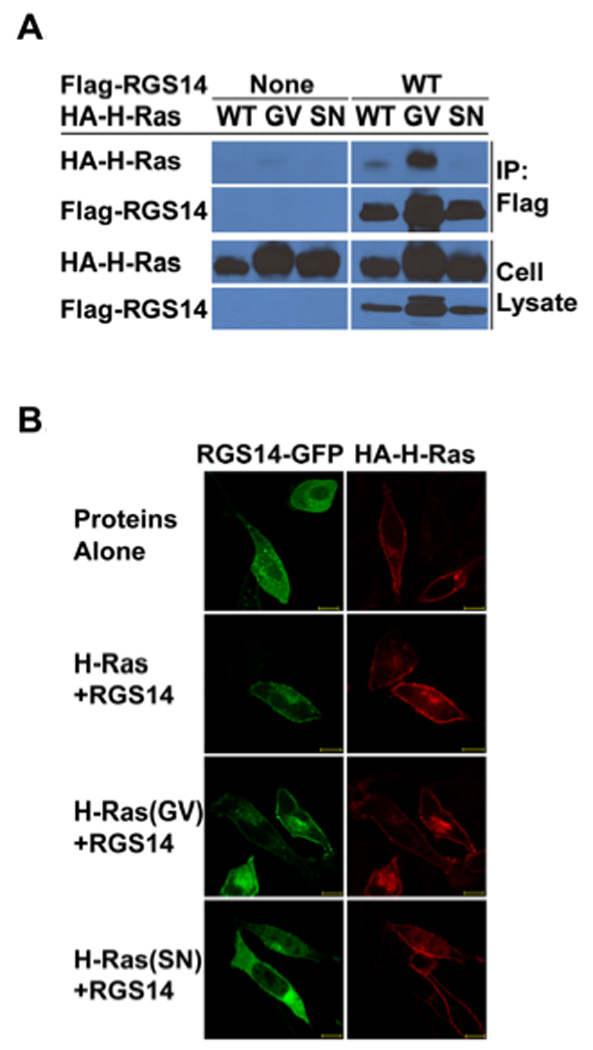
(A) RGS14 co-immunoprecipitates (co-IP) with active H-Ras from HeLa cell lysates. HeLa cells were co-transfected with Flag-tagged (Flag-RGS14) together with individual HA-tagged H-Ras (HA-H-Ras) isoforms including the activated (G/V) mutant isoform, or the inactive (S/N) mutant isoform, respectively. Whole-cell extracts were subjected to immunoprecipitation followed by immunoblot with the indicated antibodies. Cell extracts transfected with individual H-Ras isoforms alone were set as controls. The cell lysate shown in the lanes represent 5% of the whole cell extracts used for IP. (B) RGS14 co-localizes with active H-Ras(GV), but not inactive H-Ras(SN) on the plasma membrane. HeLa cells were transfected with HA-H-Ras, RGS14-GFP or RGS14-GFP together with wild type HA-H-Ras, active HA-H-Ras(G/V) or inactive HA-H-Ras(S/N), respectively. After transfection, cells were fixed, and HA-H-Ras isoforms were immunostained with Rhodamine-conjugated anti-HA antibody. Proteins were visualized by confocal microscopy as described in Experimental Procedures. RGS14 was detected by intrinsic fluorescence of GFP using confocal microscopy. Scale bars equal 10 µm. Results are representative of at least three separate experiments for each condition.
