Figure 7. RGS14 inhibition of PDGF-signaling depends on H-Ras binding and is reversed by Giα1.
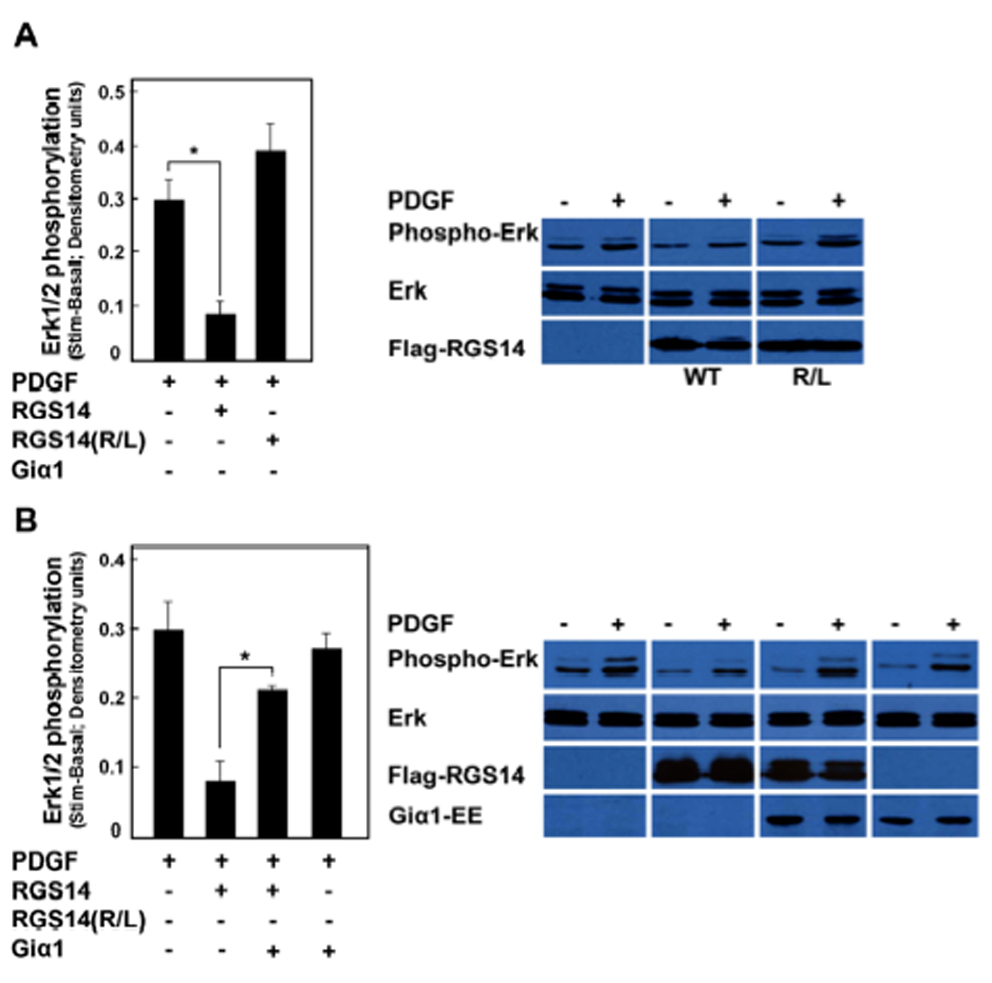
(A) RGS14 inhibition of PDGF-signaling depends on H-Ras binding. HeLa cells transfected with either empty vector (control), Flag-RGS14 or with the R333L mutant of Flag-RGS14 were stimulated with PDGF-AB (25 ng/ml) for 10 min. Phosphorylation of Erk1/2 in PDGF-AB was detected in cell lysates by immunoblot analysis using the anti-phosphoErk42/44 antibody. Results from three separate experiments were quantified by densitometry analysis and the pooled results averaged (+/− SEM) as described in Experimental Procedures. Immunoblot results are shown from a single representative experiment used for densitometry. Differences in unstimulated basal and stimulated conditions were compared and subjected to statistical analysis (paired t-test). RGS14 significantly inhibited PDGF-stimulated Erk1/2 phosphorylation (*P<0.05) but RGS14(R333L) did not. (B) RGS14 inhibition of PDGF-signalling is reversed by Giα1. HeLa cells transfected with either empty vector (control), Flag-RGS14, Flag-RGS14 and Giα1-EE together, or with Giα1-EE alone, were stimulated with PDGF-AB (25 ng/ml) for 10 min. Phosphorylation of Erk1/2 in PDGF-AB was detected in cell lysates by immunoblot analysis using the anti-phosphoErk p42/44 antibody. Results from three separate experiments were quantified by densitometry analysis and the pooled results averaged (+/− SEM) as described in Experimental Procedures. Immunoblot results are shown from a single representative experiment used for densitometry. Differences in unstimulated basal and stimulated conditions were compared and subjected to statistical analysis (paired t-test). RGS14 inhibition of PDGF-stimulated Erk1/2 phosphorylation was significantly reversed by co-expression of Giα1 (*P<0.01), whereas Giα1 alone had no effect on PDGF signalling.
