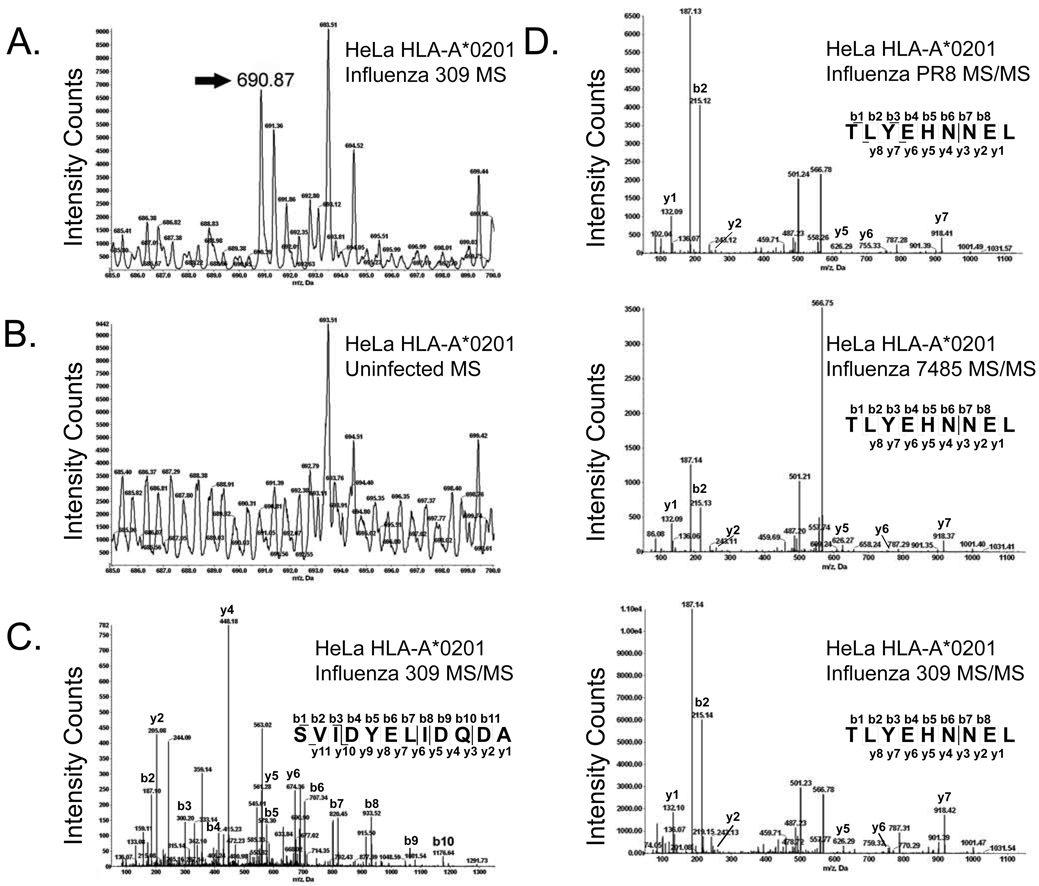
Fig. 1.
Identification of unique and increased peptides by mass spectrometry following influenza A virus infection. The annexin A2202–213 (SVIDYELIDQDA) ligand was unique to influenza 309 infected cells. An ion with the mass of 690.87 atomic mass units (amu) was identified as unique to infected cells by aligning the MS spectra of corresponding infected (A) and naïve (B) RP-HPLC fractions at 20 amu increments. Amino acid sequencing of the unique ion by MS/MS fragmentation identified a peptide derived from annexin A2 (SVIDYELIDQDA) (C). TLYEHNNEL, a nine amino acid peptide derived from the AAAS protein, was increased following infection with all three influenza A virus strains (fold increase: PR8-2.49, 7485-2.84, and 309-2.2). The amino acid sequence was determined by MS/MS fragmentation (D).