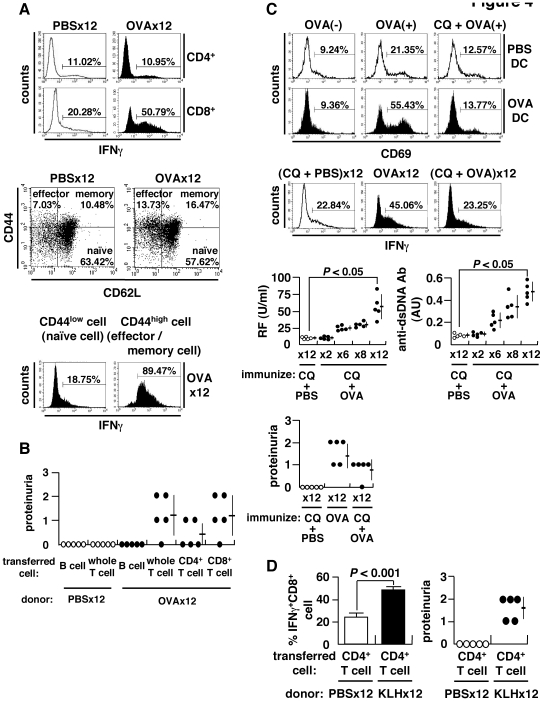
Figure 4. Expansion of CD8+ T cells and antigen cross-presentation.
(A) Spleen cells stimulated with 50 ng/ml phorbol myristate acetate (PMA) and 500 ng/ml ionomycin for 4 h in the presence of brefeldin A (10 µg/ml) and stained for intracellular IFNγ (upper). Subsets of CD8+ T cells categorized into naïve (CD44lowCD62Lhigh), effector (CD44highCD62Llow), and memory (CD44highCD62Lhigh) fractions (middle). Flow cytometry of IFNγ+ cells within naïve or effector/memory CD8+ T cell populations. Spleen cells were separated into naïve (CD44low) and effector/memory (CD44high) cells using CD44 MACS beads, and IFNγ+ cells within the CD8+ T population was evaluated (lower). (B) Adoptive transfer of splenocytes of OVA-immunized BALB/c mice into naïve recipients. The recipients were injected with 500 µg OVA 24 h after cell transfer, and proteinuria examined 2 weeks later. (C) Cross-presentation of OVA to CD8+ T cells. Splenic CD11c+ DC from OVA-immunized or control mice were incubated in the presence (OVA(+)) or absence (OVA(−)) of 1 mg/ml OVA with or without chloroquine (CQ) (20 µg/ml) for 3 h, followed by a co-culture with KJ1-26+CD8+ T cells of DO11.10 transgenic mice for 24 h to examine surface expression of CD69 (upper). Inhibition of cross-presentation in vivo by administration of 250 µg CQ per mouse 3 h prior to immunization with OVA or PBS. IFNγ+CD8+ T cells (middle), autoantibodies and proteinuria (lower) after 12× immunization. (D) Requirement of autoantibody-inducing CD4+ T cells for CD8+ T cell-mediated autoimmune tissue injury. BALB/c mice were immunized 12× with KLH, and CD4+ T cells were isolated using MACS beads. Cells were transferred into the anti-CD4 antibody-treated recipient mice immunized 8× with OVA. Percent matured CTL, i.e., IFNγ+CD8+ T cells, and proteinuria were measured 2 weeks after booster immunization 1× with KLH.