Abstract
G protein-coupled receptors (GPCRs) represent a large family of seven transmembrane receptors, which communicate extracellular signals into the cellular lumen. The human genome contains 720–800 GPCRs, and their diverse signal characteristics are determined by their specific tissue and subcellular expression profiles, as well as their coupling profile to the various G protein families (Gs, Gi, Gq, G12). The G protein coupling pattern links GPCR activation to the specific downstream effector pathways. G12/13 signalling of GPCRs has been studied only recently in more detail, and involves activation of RhoGTPase nucleotide exchange factors (RhoGEFs). Four mammalian RhoGEFs regulated by G12/13 proteins are known: p115-RhoGEF, PSD-95/Disc-large/ZO-1 homology-RhoGEF, leukemia-associated RhoGEF and lymphoid blast crisis-RhoGEF. These link GPCRs to activation of the small monomeric GTPase RhoA, and other downstream effectors. Misregulated G12/13 signalling is involved in multiple pathophysiological conditions such as cancer, cardiovascular diseases, arterial and pulmonary hypertension, and bronchial asthma. Specific targeting of G12/13 signalling-related diseases of GPCRs hence provides novel therapeutic approaches. Assays to quantitatively measure GPCR-mediated activation of G12/13 are only emerging, and are required to understand the G12/13-linked pharmacology. The review gives an overview of G12/13 signalling of GPCRs with a focus on RhoGEF proteins as the immediate mediators of G12/13 activation.
Keywords: G protein-coupled receptor, G12/13, RhoGTPase nucleotide exchange factor, RhoA, Rho kinase, fluorescence imaging
Activation of RhoGEF by G12-coupled G protein-coupled receptors (GPCRs)
Coupling to G12/13 proteins is being found for an increasing number of GPCRs, but remains to be elucidated for many others. Known GPCRs are purinergic receptors (P2Y1, P2Y2, P2Y4, P2Y6), M1 and M3 muscarinic acetylcholine receptors, receptors for thrombin [protease-activated receptor (PAR)-1, PAR-2], thromboxane (TXA2), sphingosine 1-phosphate (S1P2, S1P3, S1P4 and S1P5), lysophosphatidic acid (LPA1, LPA2, LPA3), angiotensin II (AT1), serotonin (5-HT2c and 5-HT4), somatostatin (sst5), endothelin (ETA and ETB), cholecystokinin (CCK1), V1a vasopressin receptors, D5 dopamine receptors, fMLP formyl peptide receptors, GAL2 galanin receptors, EP3 prostanoid receptors, A1 adenosine receptors, α1 adrenergic receptors, BB2 bombesin receptors, B2 bradykinin receptors, calcium-sensing receptors, KSHV-ORF74 chemokine receptors, NK1 tachykinin receptors and thyroid-stimulating hormone (TSH) receptors (Katoh et al., 1996; Sauzeau et al., 2000; Komatsuzaki et al., 2001; Thevananther et al., 2001; Greenberg et al., 2003; Zheng et al., 2003; Riobo and Manning, 2005; Hains et al., 2006; Alexander et al., 2008). G12/13 coupling can be predicted using a hidden Markov model algorithm based on the presence of certain amino acid sequences (Sgourakis et al., 2005). All known G12/13-linked GPCRs signal additionally through other types of G proteins such as Gi/o and/or Gq/11, but GPCRs uniquely coupling to G12/13 may have been missed in the past as assay technologies for their readout evolved only recently (Riobo and Manning, 2005; Siehler, 2008). Activation of G12/13-coupled GPCRs causes activation of heterotrimeric G12 and/or G13 proteins (Figure 1). The activated and guanosine triphosphate (GTP)-bound α subunit is thought to dissociate from the βγ dimer, and its activity is terminated upon GTP hydrolysis (Hepler and Gilman, 1992). Gα12 and Gα13 were discovered in 1991 as a fourth class of Gα proteins. They are ubiquitously expressed and mouse isoforms reveal 67% homology (Strathmann and Simon, 1991; Offermanns, 2003). N terminal palmitoylation is relevant for plasma membrane attachment and receptor interactions. Their slow guanosine diphosphate (GDP)/GTP exchange rate indicates their importance in prolonged signalling (Dhanasekaran and Dermott, 1996).
Figure 1.
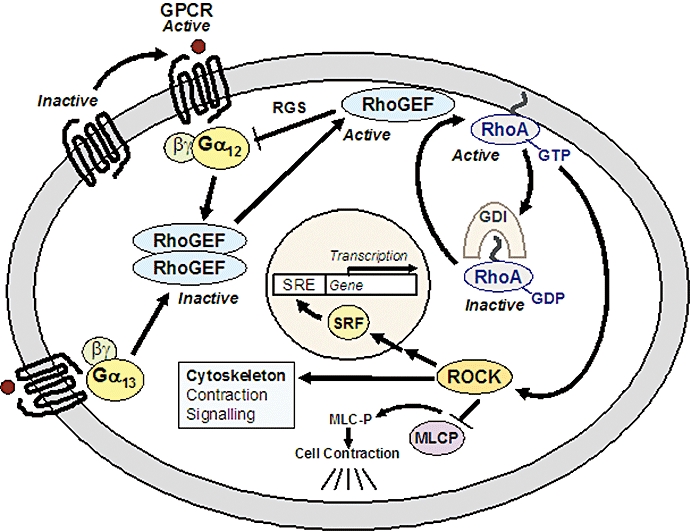
G12/13-RhoGEF-RhoA signalling pathway. GPCR-activated G12/13-proteins mediate translocation of RhoGEF from the cytosol to the plasma membrane, and stimulate its GEF activity. RhoGEFs oligomerize in their inactive form, and active monomers bind via their RGS domains to active Gα12/13 at the plasma membrane. RhoGEF reveals GAP activity towards Gα12/13, and induces downstream activation of the monomeric GTPase RhoA. Active GTP-bound RhoA is released from its inhibitory protein RhoGDI, and anchors to the plasma membrane through its geranylgeranyl-residue. Downstream targets of RhoA include ROCK, which mediates cell contraction through inhibition of MLC phosphatase, and increased SRF-dependent gene transcription. GAP, GTPase-activating protein; GDI, guanine nucleotide dissociation inhibitor; GEF, guanine nucleotide exchange factor; GPCR, G protein-coupled receptor; GTP, guanosine triphosphate; MLC, myosin light chain; RGS, regulator of G protein signaling; ROCK, Rho kinase; SRF, serum response factor.
‘Regulator of G protein signalling’ (RGS) proteins further regulate the activity of G proteins, mostly as GTPase-activating proteins (GAPs) accelerating G protein inactivation, or they can enhance G protein function (Zhong and Neubig, 2001; Xie and Palmer, 2007). RGS proteins for G12/13 proteins are RhoGEFs, four of which are known to be regulated by G12-type proteins: p115-RhoGEF, PSD-95/Disc-large/ZO-1 homology (PDZ)-RhoGEF, leukemia-associated RhoGEF (LARG) and lymphoid blast crisis (Lbc)-RhoGEF. They function as GAPs towards Gα12/13, and binding to Gα12/13 stimulates their guanine nucleotide exchange factor (GEF) activity (Fukuhara et al., 2001; Dutt et al., 2004). RhoGEF catalyses the exchange of GDP for GTP by promoting an active conformation of the small monomeric GTPase RhoA (Sah et al., 2000; Wheeler and Ridley, 2004). Upon activation by Gα12/13 RhoGEF translocates from the cytosol to the plasma membrane (Meyer et al., 2008). Oligomerization of RhoGEF negatively regulates its activity, which may prevent interaction with RhoA (Chikumi et al., 2004; Baisamy et al., 2005). Activation of RhoA is further regulated by guanine nucleotide dissociation inhibitors (GDIs), which bind to the C terminus of RhoA, where they mask the geranylgeranyl residue responsible for membrane association. RhoGDI release is required for RhoA activation, and it extracts RhoA from membranes for subsequent inactivation. Moreover, RhoGAP proteins inactivate RhoA by accelerating intrinsic GTPase activity (Fukuhara et al., 2001; Wheeler and Ridley, 2004). RhoA regulates multiple downstream effectors including many cytoskeletal proteins, and most of these have not been studied in detail. Research has elucidated RhoA-mediated activation of Rho kinases (ROCK1/2) and certain downstream pathways. ROCK phosphorylates the focal adhesion kinase (FAK), which leads to formation of actin stress fibres, and to activation of the ‘serum response’ transcription factor SRF. SRF binds in the nucleus to serum response element (SRE) sequences in the promoter of specific genes, and regulates their transcription (Narumiya et al., 1997; Treisman et al., 1998; Riento and Ridley, 2003). ROCK causes on the other hand inhibition of myosin light chain (MLC) phosphatase and direct phosphorylation of MLCs. The increase of phosphorylated MLCs in the cytoskeleton induces cell contraction (Narumiya et al., 1997; Riento and Ridley, 2003). Described cellular phenomena caused by the G12/13-RhoGEF-RhoA pathway of GPCRs hence often involve signalling or contraction through cytoskeletal proteins.
G12/13-regulated RhoGEF proteins
GPCRs coupled to G12/13 proteins activate the RhoGEF family members p115-RhoGEF, PDZ-RhoGEF, LARG and Lbc-RhoGEF (Fukuhara et al., 2001; Dutt et al., 2004). RhoGEFs are large proteins, and human p115-RhoGEF, PDZ-RhoGEF and LARG consist of 927, 1522 and 1544 amino acids respectively. Lbc-RhoGEF has multiple splice variants present in various tissues, and the unspliced form consists of 893 amino acids (Table 1). The amino acid identity of human p115-RhoGEF and human PDZ-RhoGEF, LARG and Lbc-RhoGEF is 36%, 39% and 31% respectively. All four RhoGEFs are widely expressed in mammals (Fukuhara et al., 2001; Dutt et al., 2004). High transcript levels of p115-RhoGEF are present in hematopoietic cells, and of PDZ-RhoGEF in the central nervous system (Kuner et al., 2002). The Lbc-RhoGEF splice variant A-kinase anchoring protein (AKAP)-Lbc is highly expressed in the heart. AKAPs bind the regulatory subunit type II of the cyclic adenosine monophosphate-dependent protein kinase A (PKA) anchoring it at specific subcellular sites, but additionally function as scaffold proteins for other signalling complexes (Diviani et al., 2001; 2004;).
Table 1.
Characteristics of G12/13-regulated RhoGEF Proteins
| p115-RhoGEF | PDZ-RhoGEF | LARG | Lbc-RhoGEF | |
|---|---|---|---|---|
| Expression | Blood cells, wide at low levels | CNS, wide at low levels | Ubiquitous | Wide (AKAP-Lbc in heart) |
| Size (human) | 927 aa | 1522 aa | 1544 aa | Variable (splice variants) |
| RGS domain | Yes | Yes | Yes | RGS-like (C-terminal) |
| PDZ domain | No | Yes | Yes | No |
| DH domain | Yes | Yes | Yes | Yes |
| PH domain | Yes | Yes | Yes | Yes |
| Localization inactive form | Cytoplasm | Cytoplasm or PM (cell type) | Cytoplasm or PM (cell type) | Cytoplasm |
| Localization active form | Plasma membrane | Plasma membrane | Plasma membrane | Plasma membrane |
| Gα12-induced GEF activation | No | No | Yes | Yes |
| Gα13-induced GEF activation | Yes | Yes | Yes | Yes |
| Regulation of GEF activity | PKC (+), PAK (−) | Tec, FAK (+) | Tec, FAK (+) | PKA (-) for AKAP-Lbc |
| Oligomerization | Homo-oligomers | Homo- and hetero-oligomers | Homo- and hetero-oligomers | Homo-oligomers (AKAP-Lbc) |
| Knockout mice | Yes | No | No | No |
AKAP, A-kinase anchoring protein; CNS, central nervous system; DH, Dbl-homology; FAK, focal adhesion kinase; GEF, guanine nucleotide exchange factor; LARG, leukemia-associated RhoGEF; Lbc, lymphoid blast crisis; PAK, p21-activated kinase; PDZ, PSD-95/Disc-large/ZO-1 homology; PH, pleckstrin-homology; PKA, protein kinase A; PKC, protein kinase C; PM, plasma membrane; RGS, regulator of G protein signalling; Tec, tyrosine kinase expressed in hepatocellular carcinoma.
All four RhoGEFs contain an RGS domain to directly interact with G12/13 and to exert GAP activity towards G12/13 The RGS sequence homology to classical RGS proteins is rather low. Whereas p115-RhoGEF, PDZ-RhoGEF and LARG contain the RGS domain in the N terminal sequence, Lbc-RhoGEF has a C terminal region sharing 39% amino acid identity to the consensus RGS domain and hence called ‘RGS-like’. Nevertheless, the homology of the RGS domain of p115-RhoGEF with those of PDZ-RhoGEF and LARG is also only 32% and 41% respectively (Hart et al., 1996; Kozasa et al., 1998; Wells et al., 2002; Dutt et al., 2004). Two other common motifs of RhoGEF proteins in the C terminal region are the Dbl-homology (DH) and pleckstrin-homology (PH) domains. The DH domain specifically binds to and stabilizes nucleotide- and Mg2+-free RhoA transition states to enhance its nucleotide exchange. Cells contain higher levels of GTP as compared with GDP, and the RhoGEF DH domain therefore induces loading of RhoA with GTP, and its transition into an active state (Rossman et al., 2005). The PH domain is essential for full GEF activity, and moreover anchors RhoGEF to other signalling proteins to trigger specific subcellular localizations (Fukuhara et al., 2001; Dutt et al., 2004). PDZ-RhoGEF and LARG additionally contain an N terminal PDZ domain, which enables coupling to cell surface receptors such as plexins, insulin-like growth factor receptors or GPCRs (Fukuhara et al., 2001; Taya et al., 2001; Chikumi et al., 2004; Dutt et al., 2004; Yamada et al., 2005; Grabocka and Wedegaertner, 2007; Kelly et al., 2007). The G protein-coupled LPA1 and LPA2 receptors were shown to directly interact through their C terminal PDZ domain-binding motifs with the PDZ domain of LARG and PDZ-RhoGEF to activate RhoA (Yamada et al., 2005), and it remains to be seen whether further GPCRs can directly interact with these RhoGEFs.
p115-RhoGEF localizes throughout the cytosol, and rapidly translocates to the plasma membrane upon GPCR-activation of Gα12/13, or expression of constitutively active Gα12/13 mutants (Bhattacharyya and Wedegaertner, 2003a; Meyer et al., 2008). An E229L mutation of Gα13 disrupted p115-RhoGEF interaction and recruitment to the plasma membrane (Grabocka and Wedegaertner, 2005). E27 and E29 in the acidic-rich N terminus of p115-RhoGEF are besides the RGS and PH domains required for binding to activated Gα13, but not for plasma membrane translocation (Bhattacharyya and Wedegaertner, 2003a,b;). LARG is reported to be distributed throughout the cytoplasm in most cells, except of Madin-Darby canine kidney cells, in which LARG is localized at lateral membranes. In fibroblasts, LARG was reported to localize to the microtubule-organizing centre and along microtubule tracks to contribute to cell polarity. PDZ-RhoGEF localizes in some cell types also in the cytosol, but in others at the cell periphery at or near the plasma membranes where it interacts with cortical actin. In polarizing neutrophils, PDZ-RhoGEF localizes to the back of the cells. Activation of PDZ-RhoGEF and LARG by Gα12/13 induces their translocation to the plasma membrane (Banerjee and Wedegaertner, 2004; Wong et al., 2007; Goulimari et al., 2008; Meyer et al., 2008). The subcellular localization of LARG and PDZ-RhoGEF might depend on PDZ-interacting proteins present in a cell. Gα12 and Gα13 specifically interact with distinct splice variants of Lbc-RhoGEF, and cause redistribution of Lbc-RhoGEF from the cytosol to the plasma membrane (Diviani et al., 2001; 2004;). The localization of all RhoGEFs specifically controls the spacial distribution of RhoA activity in cells.
Gα13, but not Gα12, was found to stimulate the GEF activity of p115-RhoGEF and PDZ-RhoGEF (Hart et al., 1998; Tanabe et al., 2004). Gα12 is reported to enhance the GEF activity of tyrosine-phosphorylated LARG, but not of unphosphorylated LARG. Gα13 on the other hand enhances more weakly both phosphorylated and unphosphorylated forms of LARG (Suzuki et al., 2003). Gα12/13 enhance the GEF activity of the Lbc-RhoGEF splice variant AKAP-Lbc through triggering the release of PKA, which inhibits its GEF activity by phosphorylation and concomitant recruitment of 14-3-3 (Diviani et al., 2001; Kurose, 2003; Diviani et al., 2004). Stimulation of RhoGEF activities by kinases include tyrosine phosphorylation of PDZ-RhoGEF and LARG by FAK or Tec (Fukuhara et al., 2001; Suzuki et al., 2003; Chikumi et al., 2004), and serine phosphorylation of p115-RhoGEF by protein kinase C (PKC)α (Holinstat et al., 2003). p21-activated kinase (PAK)1 can bind to the DH/PH domains of p115-RhoGEF (but not of LARG or PDZ-RhoGEF), and mediate Rac-induced inhibition of RhoA activation (Rosenfeldt et al., 2006).
RhoGEFs were recently found to oligomerize through their C terminus, which inhibits their GEF activity and interaction with RhoA. Oligomerization was not affected by interaction with Gα12/13. p115-RhoGEF and AKAP-Lbc form homooligomers, whereas PDZ-RhoGEF and LARG can form homo- and heterooligomers. Blockade of AKAP-Lbc oligomerization abolished the inhibitory effect of PKA and 14-3-3 (Chikumi et al., 2004; Baisamy et al., 2005). RhoGEFs can compete with other RGS proteins for binding to Gα12/13, which can prevent RhoA activation (Kelly et al., 2007). Examples are axin which blocks Gα12-p115-RhoGEF interaction, and RGS16 that prevents Gα13-p115-RhoGEF interaction (Johnson et al., 2003; Stemmle et al., 2006). Mutant RhoGEFs lacking GEF activity function as dominant-negative inhibitors of GPCR- or G12/13-mediated responses, whereas constitutively active p115-RhoGEF, lacking the N and C terminal regulatory domains, activates RhoA (Mao et al., 1998; Fukuhara et al., 1999; Lee et al., 2004). G12/13-regulated RhoGEFs all activate RhoA, but are not completely redundant. Using small interfering ribonucleic acid, the thrombin receptor was found to signal through LARG to activate RhoA, whereas the LPA receptor utilized PDZ-RhoGEF (Wang et al., 2004).
Only limited genetic mouse models and genetic linkages are known for the four G12/13-regulated RhoGEFs. Knockout mice are described for p115-RhoGEF, but not yet for PDZ-RhoGEF, LARG and Lbc-RhoGEF. The murine homologue of human p115-RhoGEF, Lsc, is solely expressed in hematopoietic cells. Lsc-knockout mice reveal impaired B/T lymphocyte proliferation, and B cell homing (Francis et al., 2006). Moreover, a mutant form of LARG has been found in some human cancers such as acute myeloid leukemia (Kourlas et al., 2000). In the worm species Caenorhabditis elegans only one RGS-containing RhoGEF exists, RHGF-1, that is a homologue of the mammalian p115-RhoGEF. RHGF-1 is regulated by the Gα12 homologue GPA-12, and causes activation of the RhoA homologue RHO-1. RHO-1 has been characterized to regulate neuronal activity such as acetylcholine release and locomotion (McMullan and Nurrish, 2007).
Downstream signalling of RhoGEF proteins
The G12/13-regulated RhoGEFs, p115-RhoGEF, PDZ-RhoGEF, LARG and Lbc-RhoGEF, cause activation of RhoA. Three closely related isoforms of Rho exist: RhoA, RhoB and RhoC, which are ubiquitously expressed, share 85% amino acid sequence identity, and have a C terminal geranylgeranyl residue. RhoA plays a key role in the regulation of the actin cytoskeleton, cell shape, cell polarity, microtubule dynamics, membrane transport pathways, gene transcription, cell adhesion, cell migration, neurite extension/retraction and cell growth. The roles of RhoB and RhoC are less clear. Activation of RhoA by a G12/13-linked GPCR induces its redistribution to the plasma membrane, which is reversed by injection of RhoGDI (Michaelson et al., 2001; Bhattacharya et al., 2004; Wheeler and Ridley, 2004; Wennerberg and Der, 2004; Yonemura et al., 2004; Jaffe and Hall, 2005; Rossman et al., 2005). Three RhoGDI proteins are described, and only RhoGDIα is ubiquitously expressed and has the highest affinity for RhoA. Phosphorylation of RhoGDIα by PKCα causes activation of RhoA, whereas phosphorylation by PKA causes RhoA inhibition (Mehta et al., 2001; Jaffe and Hall, 2005). Binding of GTP to RhoA causes its conformational change, and subsequent interaction with effector proteins (Bhattacharya et al., 2004). C3 toxin specifically ADP-ribosylates RhoA at N41 in the effector domain and inactivates RhoA. It is utilized to detect involvement of RhoA in signalling (Jaffe and Hall, 2005). The RhoA-S19N dominant-negative mutant has decreased affinity for GTP, and increased affinity for RhoGEFs. The GTPase activity of RhoA is impaired by G14V or G63L mutations, and these result in a constitutively active mutant (Seasholtz et al., 1999). Mutant RhoA forms do not bind RhoGDI, and localize at the plasma membrane and internal membranes (Jaffe and Hall, 2005).
Many effector proteins of RhoA have been found, but only a few have been further studied. The best characterized RhoA effectors, ROCK1/2, are ubiquitously expressed serine/threonine kinases, which translocate from the cytosol to the plasma membrane in the presence of active RhoA (Narumiya et al., 1997). Binding to RhoA disrupts the interaction between the ROCK kinase domain and its autoinhibitory C terminal domain. ROCK-mediated effects can be prevented by the selective and adenosine triphosphate-competitive ROCK inhibitor fasudil (Y27632) (Sah et al., 2000). ROCK phosphorylates many substrates such as FAK, c-Jun N-terminal kinase (JNK), MLC phosphatase, ezrin/radixin/moesin (ERM), LIM kinase (LIMK), Diaphanous (mDia), rhophilin, rhotekin, citron kinase and microtubule-associated Tau. In neurons, ROCK inactivates collapsin response mediator protein-2 (CRMP-2), which leads to blockade of microtubule assembly and hence neurite retraction (Sah et al., 2000; Jaffe and Hall, 2005). Phosphorylated FAK enhances formation of focal extracellular matrix adhesions of cells, complexes additionally containing paxillin, talin, α-actinin and vinculin, among other proteins. Focal adhesions link actin stress fibres to certain integrins at the inner surface of the plasma membrane, and p115-RhoGEF, PDZ-RhoGEF and LARG activation were demonstrated to be involved in focal adhesion formation and movement (Dubash et al., 2007; Iwanicki et al., 2007). Phosphorylated active JNK causes downstream phosphorylation of the transcription factors Jun and ATF2, and hence regulates gene transcription (Kurose, 2003). As previously described, ROCK inhibits MLC phosphatase by phosphorylation of the regulatory myosin-binding subunit of myosin phosphatase (MYPT1), which results in enzymatic inhibition. The resulting increased levels of the phosphorylated regulatory light chain MLC20 of myosin II enhances actomyosin crossbridging which leads to cell contraction in different cell types (Narumiya et al., 1997). The ERM protein family links plasma membrane proteins with the actin cytoskeleton. ERM proteins are relevant in cell adhesion, cell migration and cell division, and are stabilized by ROCK through phosphorylation (Kurose, 2003; Kelly et al., 2007).
Serum response factor-dependent gene transcription is controlled by two RhoA-dependent pathways: (i) activated ROCK/LIMK stabilize F-actin by blocking the depolymerizing factor cofilin; and (ii) active mDia1/vasodilator-stimulated phosphoprotein enhance F-actin assembly by promoting filament nucleation. The resulting G-actin depletion induces activation of SRF. The transcription factor SRF binds as a dimer to SREs together with a co-transcription factor ['ternary complex factor' (TCF) like e.g. Elk-1] regulated by the Ras/Raf/extracellular signal-regulated kinase pathway. SRF controls expression of immediate-early and muscle-specific genes, including the cytoskeletal proteins β-actin and vinculin (Marinissen and Gutkind, 2005). A SRF-dependent reporter gene assay has been invented by mutation of the TCF-binding site in the c-fos SRE, and has been widely used to indirectly detect RhoA activation. The canonical SRE core sequence CC[A/T]6GG represents the DNA-binding site for SRF. Another SRF cofactor, MAL, dissociates from cytosolic actin monomers to translocate to the nucleus upon RhoA activation. The transcription of different SRF target genes requires specific SRF cofactors (Treisman et al., 1998; Jaffe and Hall, 2005). SRF is activated not only by G12/13-linked GPCRs, active Gα12/13 proteins and RhoA, but also by overexpression of p115-RhoGEF, PDZ-RhoGEF and LARG (Seasholtz et al., 1999; Chikumi et al., 2004; Wang et al., 2004).
Gene deletion studies in mice revealed differences of G12 versus G13 (Offermanns, 1999; Kelly et al., 2007); however, no effector has been identified that is uniquely activated by either G protein. A few GPCRs apparently couple only to either G protein, such as 5-HT4 and LPA2 receptors linked to G13, or the PAR-1 receptor linked to G12. PAR-1 in another cell type, however, coupled also to G13, and these apparent specificities might be cell type or assay-dependent (Gu et al., 2002; Moers et al., 2003; Yamaguchi et al., 2003; Riobo and Manning, 2005). Differences in ligand potencies and efficacies dependent on G12 versus G13 were reported for the ‘thromboxane’ TXA2 receptor in a Gα subunit-specific guanosine-5′-O-(3′-[35S]thio)-triphosphate ([35S]GTPγS) binding assay. Ligands were only weakly active or inactive at the TXA2 receptor upon coexpression or receptor fusion of Gα12, but potent in the presence of Gα13 (Zhang et al., 2006). Not only G12 versus G13 activation, but also RhoGEF and downstream effector activation differences might be explained by ligand-effector trafficking, i.e. selective ligand potencies towards specific effector pathways.
Measurement of G12/13-dependent RhoGEF activation
Multiple assay technologies are available since many years to measure Gs-, Gi- or Gq-type signalling of GPCRs. In contrast, readouts to measure modulation of the G12/13-RhoGEF-RhoA pathway emerged only recently (Siehler, 2008). Initially, RhoA antibodies have been used to immunoprecipitate RhoA, and to measure [35S]GTPγS or [32P]GTP binding. This was hampered by the lack of specificity of available RhoA antibodies versus other small RhoGTPases, and by the small signals due to a high GTP hydrolysis rate of RhoA (Seasholtz et al., 1999). Similar assays were developed to directly detect activation of G12/13 by using specific antibodies. Stimulated cells are lysed, and [35S]GTPγS binding is measured either at immunoprecipitated Gα12/13, or at Gα12/13 immunocaptured with antibody-linked scintillation proximity assay (SPA) beads (Barr et al., 1997; Milligan, 2003; DeLapp, 2004) (Figure 2). All these methods were either not quantitative, or in the case of the [35S]GTPγS SPA assay the assay window was only limited. Another method to detect RhoA activation uses the Rho binding domain (RBD) of the effector Rhotekin to specifically extract active RhoA-GTP, and to detect levels by immunoblot detection (Ren and Schwartz, 2000). The rapid GTP hydrolysis rate and immunodetection do not allow a true quantification of signals to characterize GPCR-related pharmacology.
Figure 2.
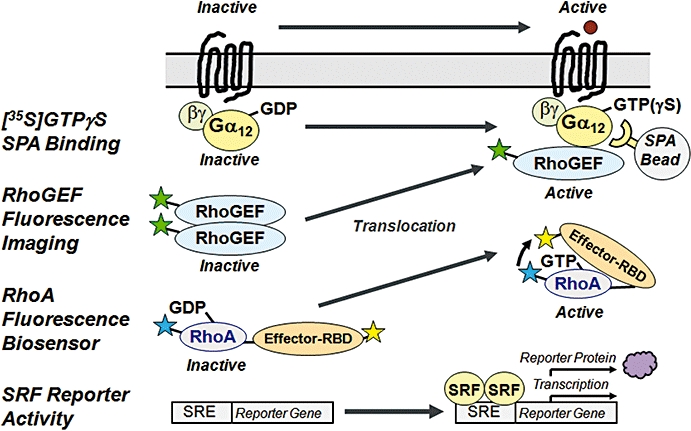
Quantitative Bioassays for Activation of the G12/13-RhoGEF-RhoA Axis. Activation of G12/13 proteins is reflected by enhanced [35S]GTPγS binding to their α subunits, which are captured by antibody-linked SPA beads. GPCR-activated G12/13 mediate redistribution of RhoGEF from the cytosol to the plasma membrane, which can be visualized by tagging of RhoGEF with, for example, EGFP (or by immunostaining). Fluorescence subcellular imaging allows quantification of cytosolic and plasma membrane-localized RhoGEF using suitable algorithms. Fluorescent biosensors contain CFP-labelled RhoA fused to the Rho binding domain (RBD) of an effector protein labelled with YFP. FRET signals between the donor CFP and acceptor YFP occur upon binding of the effector RBD to activated (GTP-bound) RhoA, and can be quantified using fluorescence imaging. Activation of RhoA can be indirectly measured in an SRF-dependent reporter gene assay. CFP, cyan fluorescent protein; EGFP, enhanced green fluorescent protein; FRET, fluorescence resonance energy transfer; GEF, guanine nucleotide exchange factor; GPCR, G protein-coupled receptor; GTP, guanosine triphosphate; GTPγS, guanosine-thio triphosphate; SPA, scintillation proximity assay; SRF, serum response factor; YFP, yellow fluorescent protein.
A quantitative method was invented using an SRF-specific reporter gene readout with a mutated c-fos promoter SRE that only binds SRF dimers but no co-transcription factor (Mao et al., 1998; Treisman et al., 1998). Nevertheless, the assay window is limited, and the RhoA-dependent activation of SRF is also modulated by other pathways. Fluorescent biosensors were developed to measure RhoA activation by detection of RhoA-GTP. One biosensor consists of four fused proteins, namely yellow fluorescent protein (YFP), the RBD of protein kinase N, truncated RhoA (amino acids 1-189) and cyan fluorescent protein (CFP). Active RhoA within the fusion protein binds the RBD of protein kinase N, and the proximity of the two fluorophores leads to an intramolecular fluorescence resonance energy transfer (FRET) signal that can be measured using subcellular confocal imaging. Another biosensor contains fused Rhotekin-RBD, CFP, YFP and C-terminal full-length RhoA, that is, the fluorophores were placed in the centre to allow RhoA to bind to RhoGDI (Yoshizaki et al., 2003; Pertz et al., 2006). These biosensors have the potential for quantitative FRET measurements using high content screening; however, only single cell analyses have been demonstrated so far.
A quantitative drug screening assay for G12/13 activation by GPCRs was recently established, and detects translocation of ‘enhanced green fluorescent protein’-tagged p115-RhoGEF. Alternatively, p115-RhoGEF can be visualized by immunostaining using a specific antibody. p115-RhoGEF translocates from the cytosol to the plasma membrane upon activation by G12/13-linked GPCRs, and signals can be recorded in a kinetic fashion. Translocation signals are measured using subcellular confocal imaging, and signals are quantified using suitable software algorithms for data analysis. All tested G12/13-coupled GPCRs mediated translocation of p115-RhoGEF. Co-expression of wildtype Gα12 or Gα13 allows to distinguish between G12- versus G13-dependent p115-RhoGEF translocation. The RhoGEF translocation assay enables compound screening and the discovery of GPCR ligands active in a G12/13-linked disease context (Meyer et al., 2008).
Dysfunction of the G12/13-RhoGEF pathway
Misregulation of G12/13 signalling of GPCRs causes multiple pathophysiological conditions, which underlines the important role of the G12/13-RhoGEF-RhoA pathway in physiological processes (Siehler, 2007; Worzfeld et al., 2008). Some of these are reviewed in more detail. In many types of smooth muscle cells (SMCs) (vascular, coronary, bronchial, cerebral, visceral and gastric) the G12-RhoA-ROCK axis causes sustained contraction through MLC phosphatase inhibition, whereas Ca2+-MLC kinase regulates transient contraction. ROCK inhibitors act on vascular SMCs to prevent vasoconstriction, and are in the clinic for arterial hypertension therapy (Watterson et al., 2005; Wettschureck and Offermanns, 2005; Brown et al., 2006). Development of arterial hypertension in a salt-induced mouse model was shown to require G12/13-LARG signalling in vascular SMCs (Wirth et al., 2008). Moreover, ROCK inhibitors are effective for treatment of cerebral vasospasm, pulmonary arterial hypertension and asthma by preventing contraction of cerebral and bronchial SMCs respectively (Calo and Pessina, 2007). Other data indicate a role of ROCK in coronary artery spasm and hence ischemic heart disease due to constriction of human coronary artery SMCs (Ohmori et al., 2003). In myocardial infarction, RhoA and ROCK expression is up-regulated and causes cardiomyocyte apoptosis, and RhoA activation induces hypertrophy of primary cardiomyocytes (Brown et al., 2006; del Re et al., 2007). Dominant-negative p115-RhoGEF blocks hypertrophic responses in cardiac myocytes (Kurose, 2003). Transgenic expression of active Gα13 or of RhoA in the heart causes cardiac hypertrophy, and p115-RhoGEF is up-regulated in hypertrophic cardiomyocytes (Wettschureck and Offermanns, 2005; Brown et al., 2006; Porchia et al., 2008).
In the immune system, the G12/13-RhoA axis is relevant in the regulation of lymphocyte adhesion and migration, and neutrophil chemokinesis and chemotaxis. Lsc/p115-RhoGEF-knockout mice reveal defective B-cell homing and T- and B-cell proliferation (Wettschureck and Offermanns, 2005; Francis et al., 2006). G12/13 signalling is required for splenic B cell maturation, migration and polarization (Rieken et al., 2006). G13 and RhoA are further required for platelet activation, shape change and aggregation, and hence for wound healing (Moers et al., 2003; Kelly et al., 2007). Constitutively active Gα12/13 proteins were found to be oncogenes in various cell types. No Gα12/13 mutations have been identified in various tumours of cancer patients, but instead increased expression of Gα12/13 and RhoA could be detected breast, prostate and colon tumours. RhoA is involved in carcinogenesis and cancer progression. Cell adhesion is reduced, which promotes cell migration and invasion of breast, prostate and colon cancer cells, and ultimatively leads to metastatic tumour progression (Kurose, 2003; Wettschureck and Offermanns, 2005; Kelly et al., 2006; 2007;). Further literature data support a role of the G12/13-RhoA axis in ovarian and lung cancer (Kelly et al., 2007; Touge et al., 2007).
The G12/13-RhoGEF-RhoA pathway of GPCRs therefore is implicated in many diseases including cardiac hypertrophy, cardiac failure, ischemic heart disease, arterial and pulmonary hypertension, bronchial asthma, inflammatory diseases, stroke, cancer progression and metastasis.
Conclusions
G12/13-signalling of GPCRs represents an emerging research area. Whereas aspects of G12/13 have been studied in greater detail in cellular systems and various tissues over the past years (Riobo and Manning, 2005; Worzfeld et al., 2008), the coupling potential of many GPCRs to this class of G proteins has often not been examined. Even less clear is the involvement of specific RhoGEF proteins and effectors in downstream signalling events. A few GPCRs were found to couple solely to G12 or G13 (Gu et al., 2002; Moers et al., 2003; Yamaguchi et al., 2003), although these specificities have been neither studied in various cell types, nor at the level of various downstream readouts. All four known G12/13-regulated RhoGEFs, p115-RhoGEF, PDZ-RhoGEF, LARG and Lbc-RhoGEF, are activated by G13. p115-RhoGEF and PDZ-RhoGEF on the other hand also translocate in the presence of GPCR-activated G12, but do not reveal any GEF activation by G12 (Hart et al., 1998; Tanabe et al., 2004; Meyer et al., 2008). Other factors such as kinases regulate the activity of RhoGEFs. Besides specificities towards G12 versus G13 regulation of RhoGEFs, two G12/13-linked GPCRs have been discovered to signal specifically through one RhoGEF versus another (Wang et al., 2004). On the other hand, no specific effector protein uniquely activated by either G12 or G13, or by either RhoGEF protein is known so far.
Understanding the complexity of the specific regulation of G12/13, RhoGEF proteins and RhoA-linked effectors is required to interpret pharmacological differences of G12/13-coupled GPCRs in various primary cell systems and tissues. Future research on the G12/13-RhoGEF-RhoA pathway of GPCRs is needed to further elucidate molecular pathways and their physiological context, which will facilitate the development of innovative therapies for patients.
Acknowledgments
The author is an employee of the Novartis Institutes for BioMedical Research.
Glossary
Abbreviations:
- AKAP
A-kinase anchoring protein
- CFP
cyan fluorescent protein
- DH
Dbl-homology
- ERM
ezrin/radixin/moesin
- FAK
focal adhesion kinase
- FRET
fluorescence resonance energy transfer
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GPCR
G protein-coupled receptor
- GTP
guanosine triphosphate
- JNK
c-Jun N-terminal kinase
- LARG
leukemia-associated RhoGEF
- Lbc
lymphoid blast crisis
- LIMK
LIM kinase
- LPA
lysophosphatidic acid
- mDia
Diaphanous
- MLC
myosin light chain
- PAR
protease-activated receptor
- PDZ
PSD-95/Disc-large/ZO-1 homology
- PH
pleckstrin-homology
- PKA
protein kinase A
- PKC
protein kinase C
- RBD
Rho binding domain
- RGS
regulator of G protein signalling
- ROCK
Rho kinase
- SMC
smooth muscle cell
- SPA
scintillation proximity assay
- SRE
serum response element
- SRF
serum response factor
- TCF
ternary complex factor
- YFP
yellow fluorescent protein
Conflict of interest
The author declares no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. (2008 revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisamy L, Jurisch N, Diviani D. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2005;280:15405–15412. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Wedegaertner PB. Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton. Mol Biol Cell. 2004;15:1760–1775. doi: 10.1091/mbc.E03-07-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AJ, Brass LF, Manning DR. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor-G protein coupling. J Biol Chem. 1997;272:2223–2229. doi: 10.1074/jbc.272.4.2223. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R, Babwah AV, Ferguson SS. Small GTP-binding protein-coupled receptors. Biochem Soc Trans. 2004;32:1040–1044. doi: 10.1042/BST0321040. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya R, Wedegaertner PB. Characterization of Ga13-dependent plasma membrane recruitment of p115RhoGEF. Biochem J. 2003a;371:709–720. doi: 10.1042/BJ20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R, Wedegaertner PB. Mutation of an N-terminal acidic-rich region of p115-RhoGEF dissociates a13 binding and a13-promoted plasma membrane recruitment. FEBS Lett. 2003b;540:211–216. doi: 10.1016/s0014-5793(03)00267-9. [DOI] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Calo LA, Pessina AC. RhoA/Rho-kinase pathway: much more than just a modulation of vascular tone. Evidence from studies in humans. J Hypertens. 2007;25:259–264. doi: 10.1097/HJH.0b013e328010d4d2. [DOI] [PubMed] [Google Scholar]
- Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, et al. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–240. doi: 10.1038/sj.onc.1207012. [DOI] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- DeLapp NW. The antibody-capture [35S]GTPγS scintillation proximity assay: a powerful emerging technique for analysis of GPCR pharmacology. Trends Pharmacol Sci. 2004;25:400–401. doi: 10.1016/j.tips.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N, Dermott JM. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- Dutt P, Nguyen N, Toksoz D. Role of Lbc RhoGEF in Gα12/13-induced signals to Rho GTPase. Cell Signal. 2004;16:201–209. doi: 10.1016/s0898-6568(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood. 2006;107:1627–1635. doi: 10.1182/blood-2005-03-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20:1661–1668. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to. Rho. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 link Gα12/13 to cell polarity and microtubule dynamics. Mol Biol Cell. 2008;19:30–40. doi: 10.1091/mbc.E06-11-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabocka E, Wedegaertner PB. Disruption of oligomerization induces nucleo-cytoplasmic shuttling of leukemia-associated RhoGEF. Mol Pharmacol. 2007;72:993–1002. doi: 10.1124/mol.107.035162. [DOI] [PubMed] [Google Scholar]
- Grabocka E, Wedegaertner PB. Functional consequences of Gα13 mutations that disrupt interaction with p115RhoGEF. Oncogene. 2005;24:2155–2165. doi: 10.1038/sj.onc.1208414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Mize GJ, Takayama TK. Protease-activated receptor mediated RhoA signaling and cytoskeletal reorganization in LNCaP cells. Biochemistry. 2003;42:702–709. doi: 10.1021/bi027100x. [DOI] [PubMed] [Google Scholar]
- Gu JL, Muller S, Mancino V, Offermanns S, Simon MI. Interaction of Gα12 with Gα13 and Gαq signaling pathways. Proc Natl Acad Sci USA. 2002;99:9352–9357. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains MD, Wing MR, Maddileti S, Siderovski DP, Harden TK. Gα12/13- and rho-dependent activation of phospholipase C-ε by lysophosphatidic acid and thrombin receptors. Mol Pharmacol. 2006;69:2068–2075. doi: 10.1124/mol.105.017921. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, et al. Direct stimulation of the guanine nucleotide exchange activity of p115RhoGEF by Gα13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Sharma S, elMasry N, Qiu RG, McCabe P, Polakis P, et al. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Cα-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner P, et al. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2007;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Seasholtz TM, Waheed AA, Kreutz B, Suzuki N, Kozasa T, et al. RGS16 inhibits signalling through the Gα13-Rho axis. Nat Cell Biol. 2003;5:1095–1103. doi: 10.1038/ncb1065. [DOI] [PubMed] [Google Scholar]
- Katoh H, Negishi M, Ichikawa A. Prostaglandin E receptor EP3 subtype induces neurite retraction via small GTPase Rho. J Biol Chem. 1996;271:29780–29784. doi: 10.1074/jbc.271.47.29780. [DOI] [PubMed] [Google Scholar]
- Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2006;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Casey PJ, Meigs TE. Biologic functions of the G12 subfamily of heterotrimeric G proteins: growth, migration, and metastasis. Biochemistry. 2007;46:6677–6687. doi: 10.1021/bi700235f. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki K, Terashita K, Kinane TB, Nishimoto I. Somatostatin type V receptor activates c-Jun N-terminal kinases via Gα12 family G proteins. Biochem Biophys Res Commun. 2001;289:1211–1217. doi: 10.1006/bbrc.2001.6085. [DOI] [PubMed] [Google Scholar]
- Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci USA. 2000;97:2145–2150. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Kuner R, Swiercz JM, Zywietz A, Tappe A, Offermanns S. Characterization of the expression of PDZ-RhoGEF, LARG and Gα12/Gα13 proteins in the murine nervous system. Eur J Neurosci. 2002;16:2333–2341. doi: 10.1046/j.1460-9568.2002.02402.x. [DOI] [PubMed] [Google Scholar]
- Kurose H. Gα12 and Gα13 as key regulatory mediator in signal transduction. Life Sci. 2003;74:155–161. doi: 10.1016/j.lfs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Lee YN, Malbon CC, Wang HY. Ga13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation. J Biol Chem. 2004;279:54896–54904. doi: 10.1074/jbc.M407581200. [DOI] [PubMed] [Google Scholar]
- McMullan R, Nurrish SJ. Rho deep in thought. Genes Dev. 2007;21:2677–2682. doi: 10.1101/gad.1615807. [DOI] [PubMed] [Google Scholar]
- Mao J, Yuan H, Xie W, Simon MI, Wu D. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J Biol Chem. 1998;273:27118–27123. doi: 10.1074/jbc.273.42.27118. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. Scaffold proteins dictate Rho GTPase-signaling specificity. Trends Sci. 2005;30:423–426. doi: 10.1016/j.tibs.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik AB. Protein kinase C-α signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Meyer BH, Freuler F, Guerini D, Siehler S. Reversible translocation of p115-RhoGEF by G12/13-coupled receptors. J Cell Biochem. 2008;104:1660–1670. doi: 10.1002/jcb.21732. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Principles: extending the utility of [35S]GTPγS binding assays. Trends Pharmacol Sci. 2003;24:87–90. doi: 10.1016/s0165-6147(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruener S, Konrad L, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Offermanns S. New insights into the in vivo function of heterotrimeric G-proteins through gene deletion studies. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:5–13. doi: 10.1007/s002109900030. [DOI] [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol. 2003;83:101–130. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Yatomi Y, Osada M, Kazama F, Takatuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S. Cardiovasc Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Ren XD, Schwartz MA. Determination of GTP loading on. Rho. Methods Enzymol. 2000;325:264–272. doi: 10.1016/s0076-6879(00)25448-7. [DOI] [PubMed] [Google Scholar]
- Porchia F, Papucci M, Gargini C, Asta A, de Marco G, Agretti P, et al. Endothelin-1 up-regulates p115RhoGEF in embryonic Rat cardiomyocytes during the hypertrophic response. J Recept Signal Transduct Res. 2008;28:265–283. doi: 10.1080/10799890802084515. [DOI] [PubMed] [Google Scholar]
- Rieken S, Sassmann A, Herroeder S, Wallenwein B, Moers A, Offermanns S, et al. G12/G13 family G proteins regulate marginal zone B cell maturation, migration, and polarization. J Immunol. 2006;177:2985–2993. doi: 10.4049/jimmunol.177.5.2985. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H, Castellone MD, Randazzo PA, Gutkind JS. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J Mol Signal. 2006;1:1–10. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, et al. P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1751–1661. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- Sgourakis NG, Bagos PG, Hamodrakas SJ. Prediction of the coupling specificity of GPCRs to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics. 2005;21:4101–4106. doi: 10.1093/bioinformatics/bti679. [DOI] [PubMed] [Google Scholar]
- Siehler S. G12/13-dependent signaling of G protein-coupled receptors: disease context and impact on drug discovery. Expert Opin Drug Discov. 2007;2:1591–1604. doi: 10.1517/17460441.2.12.1591. [DOI] [PubMed] [Google Scholar]
- Siehler S. Cell-based assays in GPCR drug discovery. Biotechnology J. 2008;4:471–483. doi: 10.1002/biot.200800001. [DOI] [PubMed] [Google Scholar]
- Stemmle LN, Fields TA, Casey PJ. The regulator of G protein signaling domain of axin selectively interacts with Gα12 but not Gα13. Mol Pharmacol. 2006;70:1461–1468. doi: 10.1124/mol.106.023705. [DOI] [PubMed] [Google Scholar]
- Strathmann MP, Simon MI. Gα12 and Gα13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci USA. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Nakamura S, Mano H, Kozasa T. Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Kreutz B, Suzuki N, Kozasa T. Regulation of RGS-RhoGEFs by Gα12 and Gα13 proteins. Methods Enzymol. 2004;390:285–294. doi: 10.1016/S0076-6879(04)90018-3. [DOI] [PubMed] [Google Scholar]
- Taya S, Inagaki N, Sengiku H, Makino H, Iwamatsu A, Urakawa I, et al. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J Cell Biol. 2001;155:809–820. doi: 10.1083/jcb.200106139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevananther S, Rivera A, Rivkees SA. A1 adenosine receptor activation inhibits neurite process formation by Rho kinase-mediated pathways. Neuroreport. 2001;12:3057–3063. doi: 10.1097/00001756-200110080-00015. [DOI] [PubMed] [Google Scholar]
- Touge H, Chikumi H, Igishi T, Kurai J, Makino H, Tamura Y, et al. Diverse activation states of RhoA in human lung cancer cells: contribution of G protein coupled receptors. Int J Oncol. 2007;30:709–715. [PubMed] [Google Scholar]
- Treisman R, Alberts AS, Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb Symp Quant Biol. 1998;63:643–651. doi: 10.1101/sqb.1998.63.643. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. Thrombin and lysophosphatidic acid receptors utilize distinct RhoGEFs in prostate cancer cells. J Biol Chem. 2004;279:28831–28834. doi: 10.1074/jbc.C400105200. [DOI] [PubMed] [Google Scholar]
- Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, et al. Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13–LARG–mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nature Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Wong K, Van Keymeulen A, Bourne H. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–1148. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T, Wettschureck N, Offermanns S. G12/G13-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci. 2008;29:582–589. doi: 10.1016/j.tips.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol. 2007;366:349–365. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Negishi M. N-terminal short sequences of alpha subunits of the G12 family determine selective coupling to receptors. J Biol Chem. 2003;278:14936–14939. doi: 10.1074/jbc.M301409200. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, DiLizio C, Kim D, Smyth EM, Manning DR. The G12 family of G proteins as a reporter of thromboxane A2 receptor activity. Mol Pharmacol. 2006;69:1433–1440. doi: 10.1124/mol.105.019703. [DOI] [PubMed] [Google Scholar]
- Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, et al. G12- and G13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–660. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- Zhong H, Neubig RR. Regulator of G protein signaling proteins: novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837–845. [PubMed] [Google Scholar]


